created: 2026-01-31
論文1:Preclinical Safety Evaluation of BB106, a Novel Ketamine-Sulfobutylether-Beta-Cyclodextrin Salt, Following Subcutaneous Administration in Rats and Göttingen Minipigs
- 著者:Ramot, Y; Andreini, I; Sacco, G; Becker, J; Bruhn, K; Manno, RA; Nyska, A
- 掲載誌:INTERNATIONAL JOURNAL OF TOXICOLOGY(2026)
- 種別:Article; Early Access
- キーワード:ketamine; subcutaneous administration; preclinical toxicology; minipig; rat; sulfobutylether-beta-cyclodextrin; SBECD; cyclodextrin; local tolerability; drug formulation; safety pharmacology
- DOI:10.1177/10915818251414705
何の研究?(ひとことで)
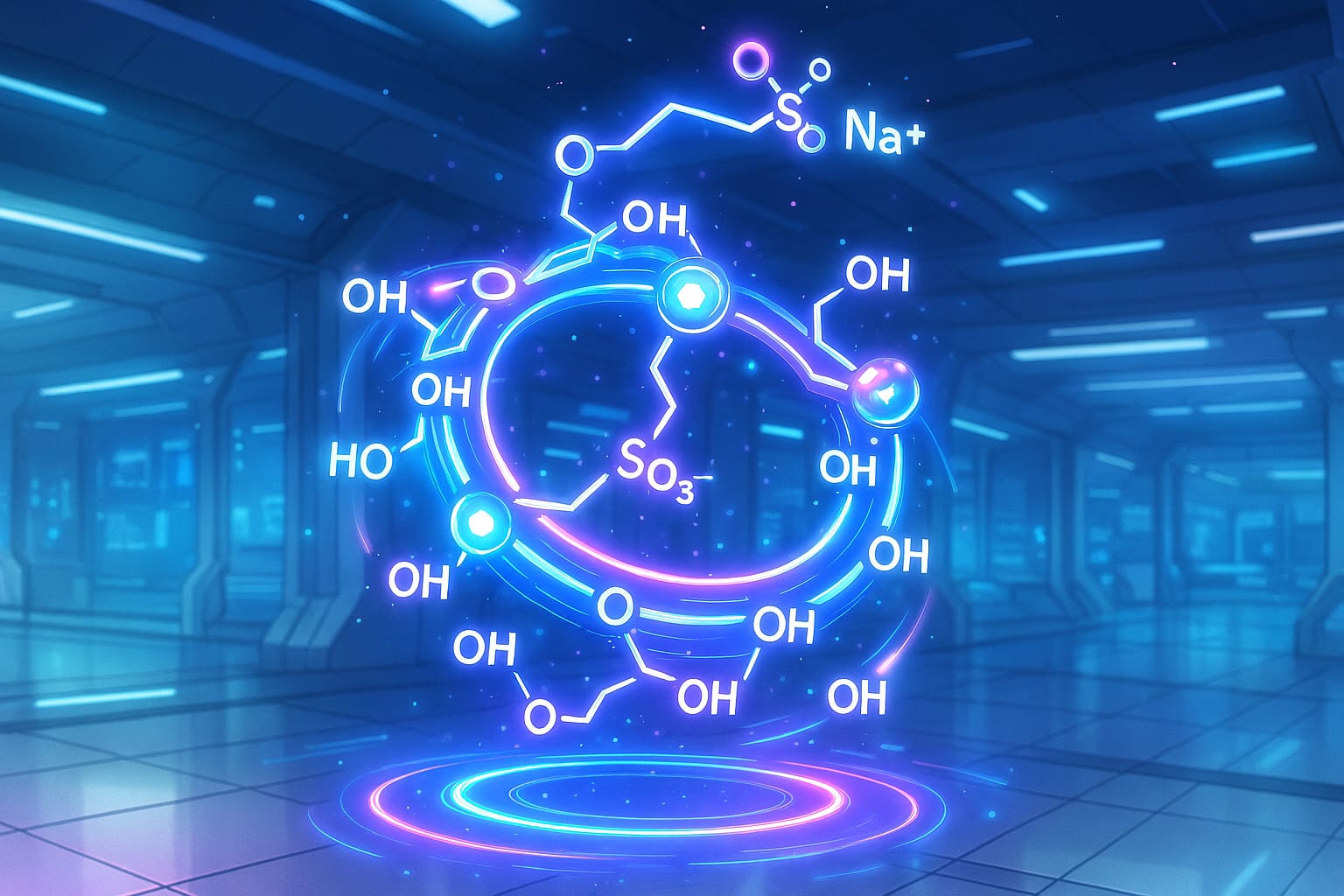
酸性・高浸透圧で皮下投与に不向きなケタミン製剤を、SBEβCD(スルホブチルエーテルβCD)塩で“ほぼ生理的pH・等張”にし、ラットとミニブタで安全性を検証した前臨床研究
ポイント(やさしく)
- 従来のケタミン塩酸塩溶液は酸性・高浸透圧になりやすく、皮下(SC)投与の刺激性が課題になり得る。
- BB106はSBEβCDの多価陰イオン置換を“対イオン”として利用し、ケタミン–SBEβCD塩として近いpH・等張性を実現。
- ラット(2週間反復皮下投与)とゲッチンゲン・ミニブタ(4週間連続皮下投与)で、局所反応は軽度かつ可逆、全身毒性の所見は限定的で、薬物動態も蓄積なしと報告。
応用イメージ(どこに役立つ?)
- 疼痛・精神神経領域での“静脈投与以外”の選択肢(皮下投与など)の実現可能性評価
- CD誘導体(SBEβCD)を用いた“刺激性を下げた薬物製剤”設計の具体例
- 安全性データに基づく臨床開発(投与経路・用量設定)の基礎資料
用語ミニ解説
- SBEβCD(Sulfobutylether‑β‑cyclodextrin):β-シクロデキストリンをスルホブチルエーテル化した誘導体。水溶性や電荷を利用して、薬物の溶解性・製剤特性を改善できる。
- 皮下投与(SC):皮膚の下に薬剤を投与する方法。刺激性や浸透圧・pHの設計が重要になる。
- 等張(Isotonicity):体液に近い浸透圧。注射時の刺激軽減に関係する。
参考文献(添付ファイル内)
- Ramot, Y; Andreini, I; Sacco, G; Becker, J; Bruhn, K; Manno, RA; Nyska, A. Preclinical Safety Evaluation of BB106, a Novel Ketamine-Sulfobutylether-Beta-Cyclodextrin Salt, Following Subcutaneous Administration in Rats and Göttingen Minipigs. INTERNATIONAL JOURNAL OF TOXICOLOGY (2026). DOI: 10.1177/10915818251414705
English
Paper 1: Preclinical Safety Evaluation of BB106, a Novel Ketamine-Sulfobutylether-Beta-Cyclodextrin Salt, Following Subcutaneous Administration in Rats and Göttingen Minipigs
- Authors: Ramot, Y; Andreini, I; Sacco, G; Becker, J; Bruhn, K; Manno, RA; Nyska, A
- Journal: INTERNATIONAL JOURNAL OF TOXICOLOGY (2026)
- Type: Article; Early Access
- Keywords: ketamine; subcutaneous administration; preclinical toxicology; minipig; rat; sulfobutylether-beta-cyclodextrin; SBECD; cyclodextrin; local tolerability; drug formulation; safety pharmacology
- DOI: 10.1177/10915818251414705
One‑sentence summary
Preclinical safety of a ketamine–SBEβCD salt (BB106): enabling near‑physiologic, isotonic subcutaneous administration
Key points
- Conventional ketamine HCl solutions can be acidic and hyperosmotic, which may limit tolerability for subcutaneous (SC) delivery.
- BB106 uses sulfobutylether‑β‑cyclodextrin (SBEβCD) anionic substitutions as counterions to form a ketamine–SBEβCD salt at near‑physiologic pH and isotonicity.
- Repeated‑dose rat studies and continuous SC infusion in Göttingen minipigs reported favorable local and systemic safety profiles with minimal, reversible injection‑site changes and no systemic target‑organ lesions.
Possible applications
- Supporting development of SC ketamine alternatives for pain and neuropsychiatric indications
- A concrete formulation strategy using cyclodextrin derivatives to improve tolerability
- Nonclinical safety package informing clinical route/dose decisions
Reference (from the provided file)
- Ramot, Y; Andreini, I; Sacco, G; Becker, J; Bruhn, K; Manno, RA; Nyska, A. Preclinical Safety Evaluation of BB106, a Novel Ketamine-Sulfobutylether-Beta-Cyclodextrin Salt, Following Subcutaneous Administration in Rats and Göttingen Minipigs. INTERNATIONAL JOURNAL OF TOXICOLOGY (2026). DOI: 10.1177/10915818251414705