date: 2026-01-12
tags: [Spiropyran, スピロピラン, Photochromism, フォトクロミズム, Merocyanine, 結晶多形, Perovskite]
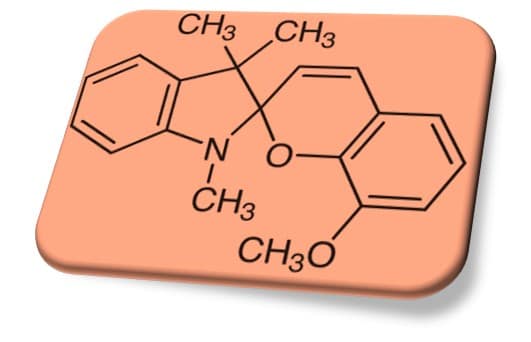
SPMt(トリメチルインドリノ系スピロピラン)とは?
スピロピラン(Spiropyran, SP)は、光で構造が切り替わり色が変わる分子スイッチです。とくに 1,3,3-トリメチルインドリノ系(本記事では便宜上「SPMt」)は、古典的な基本骨格としてよく使われ、置換基(メトキシ、ニトロなど)や相互作用相手(酸、金属塩、固体結晶)によって性質が大きく変わります。
一般向けにやさしく解説します。
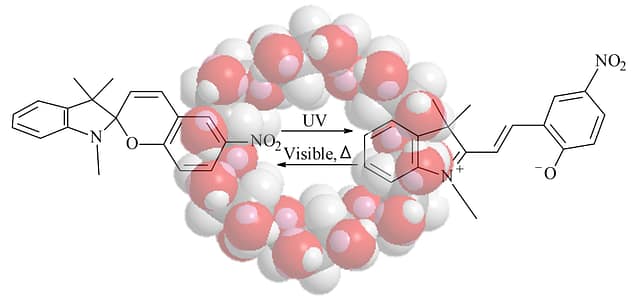
まず押さえる基本:SP ⇄ MC の切り替え
- SP(閉環体):無色〜淡色のことが多い
- MC(開環体:メロシアニン):可視域に吸収を持ち有色になりやすい
- 光(UV/可視)や周囲環境で、SP⇄MCが可逆に起こる(条件依存)
研究が進む3つの理由(ざっくり)
- 固体での“固定化”:溶液では起こる反応が固体では止まる/逆に固体で安定化できる
- 結晶多形(ポリモルフ):同じ分子でも結晶の組み方で性質が変わる
- 材料・デバイス応用:界面保護、欠陥パッシベーション、色調設計などに利用できる
代表3論文のポイント
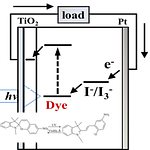
1) 光で“動的”に欠陥を埋める:無機ペロブスカイト太陽電池への応用
何をした研究?
光異性化するスピロピラン誘導体(OMe-SP)を、CsPbI₃₋ₓBrₓ 系の無機ペロブスカイト太陽電池の界面保護層として導入し、動作中に生じる欠陥も含めて「継続的に」抑える発想を示しています。
ここがポイント
– 光で状態が切り替わる分子を使い、静的ではなく“動的”な欠陥パッシベーションを狙う
– ハロゲンイオン移動を抑え、界面再結合を低減 → 劣化を遅らせる
– 例として、PCE 22.20%、MPP追従+1 sun連続照射で1032時間後に初期の91%維持(著者要約より)
一般向けに一言
「光で形が変わる分子を、太陽電池の表面に置いて“傷(欠陥)”が増えにくい状態を保つ」イメージです。
2) 結晶でわかる“固体中の姿”:スピロピラン/スピロオキサジンの構造解析
何をした研究?
市販のスピロピラン/スピロオキサジンについて、単結晶X線回折で構造を調べ、条件を変えた蒸発実験から複数の新しい結晶構造(多形を含む)を報告しています。
ここがポイント
– 5つの新規構造を取得(蒸発条件を変えて多形を探索)
– SPMt骨格は強い水素結合を作りにくい → 追加の官能基が相互作用を左右
– 結果は、関連構造のデータベース検索とも整合(著者要約より)
一般向けに一言
「同じ分子でも“並び方(結晶の組み方)”が変わると、色や反応性が変わる。その並び方を“目で見える形”で確かめた研究」です。
3) “色を設計する道具”:金属塩で固体中の色(吸収)をチューニング
何をした研究?
金属塩(ZnCl₂、ZnBr₂、CoCl₂)を用いて、固体中でMCを安定化し、スピロピランの固体吸収(色)を広い範囲で調整できることを示しています。
ここがポイント
– 固体中で稀にしか見えない有色MCを、金属塩導入で安定化
– 相互作用は錯体/塩/混合型など複数 → 合成経路の違いが効く
– 固体形の違いが吸収特性に大きく影響し、赤〜緑まで色調をカバー(著者要約より)
一般向けに一言
「“金属塩を混ぜる”ことで、固体のまま色を狙って作れる可能性を示した“配色ツール”の研究」です。
用語ミニ解説
- フォトクロミズム:光で化学構造が変わり、色(吸収)が変化する現象
- メロシアニン(MC):SPが開環した有色体。固体では安定化が難しいことが多い
- 結晶多形(ポリモルフ):同一分子でも結晶構造(並び方)が異なる状態
- 欠陥パッシベーション:材料中の欠陥を“埋める/無害化する”ことで性能低下を抑える考え方
参考文献(添付ファイル内)
- Wang ZT, Chen QY, Xie HD et al.. Light-Driven Dynamic Defect-Passivation for Efficient Inorganic Perovskite Solar Cells. Advanced Functional Materials (2025) 35(9) . https://doi.org/10.1002/adfm.202416118
- Seiler VK, Tumanov N, Robeyns K et al.. A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs. Crystals (2017) 7(3) Article 84. https://doi.org/10.3390/cryst7030084
- Seiler VK, Robeyns K, Tumanov N et al.. A coloring tool for spiropyrans: solid state metal-organic complexation versus salification. Crystengcomm (2019) 21(33) 4925-4933. https://doi.org/10.1039/c9ce00805e
English
What is SPMt (trimethylindolino spiropyran)?
Spiropyran (SP) is a classic light-driven molecular switch whose structure (and often color) can be reversibly changed.
Here, we use “SPMt” as a convenient label for 1,3,3-trimethylindolino spiropyran scaffolds, which are widely used and tunable via substituents (e.g., methoxy, nitro) and interactions (acids, metal salts, and crystal packing).
This HP-ready page highlights three papers found in the attached Web of Science export.
(Only information contained in the attached file is used.)
The basic switch: SP ⇄ MC
- SP (closed form): typically colorless/pale
- MC (open form, merocyanine): often colored (visible absorption)
- UV/visible light and the surrounding environment can drive reversible switching (condition-dependent)
Why these studies matter (quick view)
- Solid-state stabilization: switching can be suppressed or, conversely, stabilized in crystals/solids
- Polymorphism: different crystal packings of the same molecule can change properties
- Materials & devices: interface protection, defect passivation, and color tuning become possible
Three representative papers (easy highlights)
1) Light-driven dynamic defect passivation for inorganic perovskite solar cells
What was done?
A photoisomerizable spiropyran derivative (OMe-SP) was used as an interfacial protective layer on CsPbI₃₋ₓBrₓ all-inorganic perovskite solar cells, aiming at continuous (“dynamic”) passivation during operation.
Key points
– A photoswitchable molecule is used for sustainable, operation-relevant passivation rather than short-term static treatment
– Suppresses halide ion migration and reduces interfacial recombination → retards degradation
– Reported performance/stability in the abstract includes PCE 22.20% and 91% retention after 1032 h under MPP tracking and continuous 1-sun illumination (authors’ summary)
One-line takeaway
A photoswitch molecule is placed at the device interface to keep defects and ion migration under control during real operation.
2) Seeing the solid-state “arrangement”: structural analysis and polymorphs of SP/SO compounds
What was done?
Single-crystal X-ray diffraction was used to investigate commercial spiropyrans and spirooxazines, obtaining multiple new structures through isothermal evaporation under different conditions.
Key points
– Five new structures were obtained (including polymorph-related outcomes)
– The core scaffold lacks strong H-bond donors/acceptors; additional functional groups shape intermolecular interactions
– Findings are supported by a related-structure search in a crystal structure database (authors’ summary)
One-line takeaway
The study shows how changing crystal packing (polymorphs) and functional groups affects interactions in the solid state.
3) A “coloring tool” in solids: metal salts stabilize MC and tune absorption
What was done?
Metal salts (ZnCl₂, ZnBr₂, CoCl₂) were introduced to several spiropyrans to stabilize the colored merocyanine form in the solid state and tune absorption.
Key points
– Colored MC is rarely observed in solids, but metal salts help stabilize merocyanine
– Interactions can be complex/salt/mixed depending on synthetic pathways
– Solid-form control strongly impacts absorption, covering a wide visible range (reported from red to green)
One-line takeaway
Metal salts can act as a practical handle to tune solid-state color/absorption of spiropyran systems.
Mini glossary
- Photochromism: light-induced structural change with a color/absorption change
- Merocyanine (MC): the colored, ring-open form of spiropyran
- Polymorphism: multiple crystal packings for the same molecule
- Defect passivation: suppressing or neutralizing defects that reduce performance
References (from the attached file)
- Wang ZT, Chen QY, Xie HD et al.. Light-Driven Dynamic Defect-Passivation for Efficient Inorganic Perovskite Solar Cells. Advanced Functional Materials (2025) 35(9) . https://doi.org/10.1002/adfm.202416118
- Seiler VK, Tumanov N, Robeyns K et al.. A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs. Crystals (2017) 7(3) Article 84. https://doi.org/10.3390/cryst7030084
- Seiler VK, Robeyns K, Tumanov N et al.. A coloring tool for spiropyrans: solid state metal-organic complexation versus salification. Crystengcomm (2019) 21(33) 4925-4933. https://doi.org/10.1039/c9ce00805e