date: 2026-01-12
tags: [Spiropyran, SPNO2, Photochromism, Merocyanine, Crystal engineering, Acidochromism]
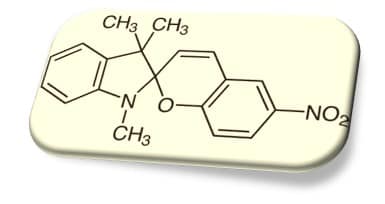
SPNO2(ニトロ置換スピロピラン)とは?
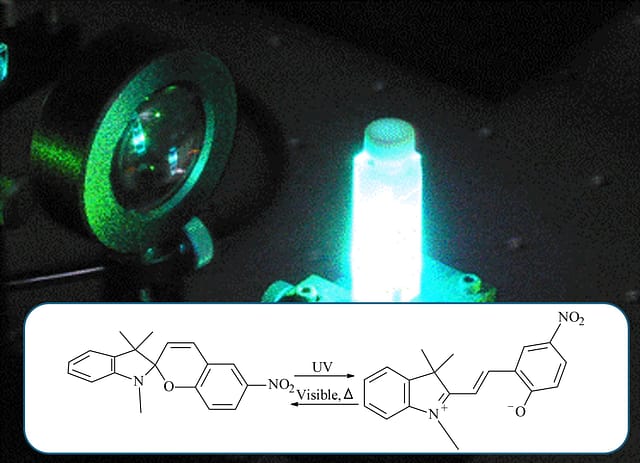
スピロピラン(spiropyran)は、光を当てると色が変わる分子として有名です。
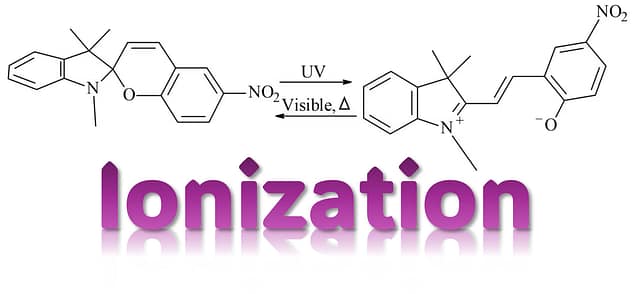
閉環体(SP:無色〜淡色)⇄ 開環体(MC:メロシアニン、着色)という「形の切り替え」で色が変わります。
その中でも SPNO2(ニトロ置換スピロピラン) は、置換基(–NO₂)の影響で電荷分布が変わり、“開いた色(MC)”の性質や固体中での安定性を議論しやすい代表例として多く扱われます。
ここがポイント(一般向け)
- 液体中では色が変わりやすい一方で、固体では分子が動きにくく、MC(開環体)がすぐ戻ってしまう/そもそも出にくいことがあります。
- 研究の狙いは、「固体の中で色を出したまま保つ」「環境(酸・塩・金属)で色の出方をデザインする」ことです。
- そのために使われる“鍵”が、結晶構造(分子の並び)、酸(プロトン)、金属塩です。
代表的な3報
1) 結晶構造と“多形(ポリモルフ)”が色のスイッチを左右する
狙い:スピロピラン/スピロオキサジンが固体でどう並ぶと、どんな性質になるかを整理。
何が新しい?:単結晶X線解析で、複数の光クロミック分子の結晶構造や多形を比較し、
「スピロ系分子は水素結合が作りにくい → 置換基や結晶の詰まり方が効く」ことを示します。
HP向け一言:“同じ分子でも結晶の作り方で性質が変わる(多形)” という固体化学の面白さがよく分かります。
参考:Seiler, VK, Tumanov, N, Robeyns, K, Wouters, J, Champagne, B, Leyssens, T — “A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs” — Crystals — 7 — Vol. 3 — DOI: http://dx.doi.org/10.3390/cryst7030084
2) “酸”で開環体(メロシアニン)を固体で安定化:アシドクロミズム
狙い:固体中では珍しいMC(開環体)を、無機酸との共結晶化で安定化する。
何が新しい?:スピロピラン誘導体(SPNO2 など)を酸と一緒に結晶化させ、
開環→プロトン化したMCを塩(サルティフィケーション)として固定し、色を保つ設計を示します。
HP向け一言:“酸を使うと、固体でも色を出したままにできる” というデザイン指針を与えます。
参考:Seiler, VK, Callebaut, K, Robeyns, K, Tumanov, N, Wouters, J, Champagne, B, Leyssens, T — “Acidochromic spiropyran-merocyanine stabilisation in the solid state” — Crystengcomm — 2018 — Vol. 20 — pp. 3318–3327 — DOI: 10.1039/c8ce00291f
3) 金属塩 vs 酸:固体で“色を塗り分ける”ためのツール化
狙い:金属塩(ZnCl₂, ZnBr₂, CoCl₂ など)や酸を使い分け、固体の発色を設計する。
何が新しい?:スピロピランが開環してできるMCが、
– 金属との相互作用(錯形成)
– 酸との塩形成(サルティフィケーション)
でそれぞれ違う形で安定化し、色(吸収)の範囲を広げられることを示します。
HP向け一言:“材料に混ぜる添加剤で、色を狙って作れる” という実装のイメージに繋がります。
参考:Seiler, VK, Robeyns, K, Tumanov, N, Cincic, D, Wouters, J, Champagne, B, Leyssens, T — “A coloring tool for spiropyrans: solid state metal-organic complexation versus salification” — Crystengcomm — 2019 — Vol. 21 — pp. 4925–4933 — DOI: 10.1039/c9ce00805e
用語ミニ解説
- スピロピラン(SP):閉環体。多くは無色〜淡色。
- メロシアニン(MC):開環体。共役が伸びて色が出やすい。
- アシドクロミズム:酸(プロトン)で色が変わる性質。
- 共結晶化(co-crystallization):分子同士(酸など)を一緒に結晶にして、性質を作り込む。
- 多形(polymorph):同じ分子でも結晶の並びが違う別形。性質が変わることがある。
関連文献
本文で解説した3報
- Seiler, VK, Callebaut, K, Robeyns, K, Tumanov, N, Wouters, J, Champagne, B, Leyssens, T — “Acidochromic spiropyran-merocyanine stabilisation in the solid state” — Crystengcomm — 2018 — Vol. 20 — pp. 3318–3327 — DOI: 10.1039/c8ce00291f
- Seiler, VK, Tumanov, N, Robeyns, K, Wouters, J, Champagne, B, Leyssens, T — “A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs” — Crystals — 7 — Vol. 3 — DOI: http://dx.doi.org/10.3390/cryst7030084
- Seiler, VK, Robeyns, K, Tumanov, N, Cincic, D, Wouters, J, Champagne, B, Leyssens, T — “A coloring tool for spiropyrans: solid state metal-organic complexation versus salification” — Crystengcomm — 2019 — Vol. 21 — pp. 4925–4933 — DOI: 10.1039/c9ce00805e
そのほか(同ファイル内・関連)
- Wang, ZT, Chen, QY, Xie, HD, Feng, XL, Du, YC, Zhou, TX, Li, R, Zhang, JQ, Zhang, L, Xu, Z, Xi, LL, Tian, QW, Liu, SZ — “Light-Driven Dynamic Defect-Passivation for Efficient Inorganic Perovskite Solar Cells” — Advanced Functional Materials — 2025 — Vol. 35 — DOI: 10.1002/adfm.202416118
- Hayashi, K, Watanabe, H, Imai, H — “Stable ring-opened forms of a spiropyran in the confined space of nanoporous silicas” — Microporous And Mesoporous Materials — 2024 — Vol. 371 — DOI: 10.1016/j.micromeso.2024.113084
- Takeshita, T, Yano, A, Hara, M — “Light-Driven Proton Release of Spirobenzopyran-Derived Protonated Photomerocyanine in Cyclodextrin Aqueous Solution” — Chemistryselect — 2017 — Vol. 2 — pp. 11288–11292 — DOI: 10.1002/slct.201702119
English (for your website)
What is SPNO2 (nitro-substituted spiropyran)?
Spiropyrans are well-known molecules that change color when exposed to light.
Their color switching comes from a structural interconversion:
closed form (SP; typically colorless/pale) ⇄ open form (MC; merocyanine, colored).
Among them, SPNO2 (nitro-substituted spiropyran) is frequently discussed because the –NO₂ group strongly affects charge distribution, making it a useful representative case to study the ‘open colored form’ (MC) and its stability in solids.
Key takeaways (for a general audience)
- In liquids, spiropyrans often switch colors easily, but in solids the molecular motion is restricted, so the open form can be hard to generate or quickly revert.
- A central goal is to keep the colored open form stable in the solid state, and to design color responses using the surrounding environment.
- Three practical “keys” appear repeatedly: crystal packing (structure), acids (protons), and metal salts.
Three representative papers (selected from the attached file)
1) Crystal packing and polymorphs can control the switching behavior
Aim: Organize how spiropyrans/spirooxazines pack in crystals and how polymorphs arise.
What’s new? Single-crystal X-ray diffraction is used to compare multiple structures and show why
spiropyran-type molecules often have limited hydrogen-bonding options—so substituents and packing become decisive.
One-line for your website: Even the same molecule can behave differently depending on its crystal form (polymorph).
Ref: Seiler, VK, Tumanov, N, Robeyns, K, Wouters, J, Champagne, B, Leyssens, T — “A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs” — Crystals — 7 — Vol. 3 — DOI: http://dx.doi.org/10.3390/cryst7030084
2) Acid-assisted stabilization of the open merocyanine in solids (acidochromism)
Aim: Stabilize the rarely observed open-ring merocyanine (MC) in the solid state via co-crystallization with inorganic acids.
What’s new? By forming protonated MC salts (including cases involving SPNO2 derivatives), the colored form is “locked” in crystals,
and stability against photodegradation can improve in specific cases.
One-line for your website: Acids can help keep the colored open form stable in solids.
Ref: Seiler, VK, Callebaut, K, Robeyns, K, Tumanov, N, Wouters, J, Champagne, B, Leyssens, T — “Acidochromic spiropyran-merocyanine stabilisation in the solid state” — Crystengcomm — 2018 — Vol. 20 — pp. 3318–3327 — DOI: 10.1039/c8ce00291f
3) A practical “coloring tool”: metal complexation vs salt formation
Aim: Compare metal-salt interactions (e.g., ZnCl₂, ZnBr₂, CoCl₂) with acid-driven salification to design solid-state colors.
What’s new? The study demonstrates that different synthetic pathways and interactions can stabilize merocyanine in distinct ways,
expanding the accessible absorption (color) range across the visible spectrum.
One-line for your website: Additives (acids or metal salts) can tune and broaden the solid-state color palette.
Ref: Seiler, VK, Robeyns, K, Tumanov, N, Cincic, D, Wouters, J, Champagne, B, Leyssens, T — “A coloring tool for spiropyrans: solid state metal-organic complexation versus salification” — Crystengcomm — 2019 — Vol. 21 — pp. 4925–4933 — DOI: 10.1039/c9ce00805e
Mini glossary
- Spiropyran (SP): closed form, often colorless/pale.
- Merocyanine (MC): open form, extended conjugation, typically colored.
- Acidochromism: color change driven by acids/protons.
- Co-crystallization: crystallizing two (or more) components together to engineer properties.
- Polymorph: a different crystal packing of the same molecule; properties may change.
References (from the attached file only)
The three papers explained above
- Seiler, VK, Callebaut, K, Robeyns, K, Tumanov, N, Wouters, J, Champagne, B, Leyssens, T — “Acidochromic spiropyran-merocyanine stabilisation in the solid state” — Crystengcomm — 2018 — Vol. 20 — pp. 3318–3327 — DOI: 10.1039/c8ce00291f
- Seiler, VK, Tumanov, N, Robeyns, K, Wouters, J, Champagne, B, Leyssens, T — “A Structural Analysis of Spiropyran and Spirooxazine Compounds and Their Polymorphs” — Crystals — 7 — Vol. 3 — DOI: http://dx.doi.org/10.3390/cryst7030084
- Seiler, VK, Robeyns, K, Tumanov, N, Cincic, D, Wouters, J, Champagne, B, Leyssens, T — “A coloring tool for spiropyrans: solid state metal-organic complexation versus salification” — Crystengcomm — 2019 — Vol. 21 — pp. 4925–4933 — DOI: 10.1039/c9ce00805e
Additional related records (same attached file)
- Wang, ZT, Chen, QY, Xie, HD, Feng, XL, Du, YC, Zhou, TX, Li, R, Zhang, JQ, Zhang, L, Xu, Z, Xi, LL, Tian, QW, Liu, SZ — “Light-Driven Dynamic Defect-Passivation for Efficient Inorganic Perovskite Solar Cells” — Advanced Functional Materials — 2025 — Vol. 35 — DOI: 10.1002/adfm.202416118
- Hayashi, K, Watanabe, H, Imai, H — “Stable ring-opened forms of a spiropyran in the confined space of nanoporous silicas” — Microporous And Mesoporous Materials — 2024 — Vol. 371 — DOI: 10.1016/j.micromeso.2024.113084
- Takeshita, T, Yano, A, Hara, M — “Light-Driven Proton Release of Spirobenzopyran-Derived Protonated Photomerocyanine in Cyclodextrin Aqueous Solution” — Chemistryselect — 2017 — Vol. 2 — pp. 11288–11292 — DOI: 10.1002/slct.201702119
Note: This page was generated only from the records contained in the attached Web of Science export (SPNO2_Classics.txt).