Dye-sensitied solar cell and Spyropirans
| Authors | Author Full Names | Article Title | Source Title | Author Keywords | Keywords Plus | Abstract | Addresses | Affiliations | Reprint Addresses | Email Addresses | Researcher Ids | ORCIDs | Funding Orgs | Funding Name Preferred | Funding Text | Cited References | Cited Reference Count | Times Cited, WoS Core | Times Cited, All Databases | 180 Day Usage Count | Since 2013 Usage Count | Publisher | Publisher City | Publisher Address | ISSN | eISSN | ISBN | Journal Abbreviation | Journal ISO Abbreviation | Publication Date | Publication Year | Volume | Issue | Part Number | Start Page | End Page | Article Number | DOI | DOI Link | Book DOI | Early Access Date | Number of Pages |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeshita, T; Umeda, T; Hara, M | Takeshita, Tatsuya; Umeda, Takao; Hara, Michihiro | Fabrication of a dye-sensitized solar cell containing a noncarboxylated spiropyran-derived photomerocyanine with cyclodextrin | JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY | Dye-sensitized solar cell; Photomerocyanine; Cyclodextrin; Inclusion complex; Isomerization | NANOPARTICLES | The fabrication, photovoltaic conversion, and photo-response of dye-sensitized solar cell (DSSC) containing a noncarboxylated spiropyran 1,3,3-trimethylindolino-beta-naphthopyrylospiran (1) and carboxymethy1-beta-cyclodextrin sodium salt (CM-beta-CD) were investigated for the first time. In fact, we found the adsorption of photomerocyanine form (PMC) of 1 and inclusion complexes between the PMC and CM-beta-CD (PMC/CM-beta-CD) to the TiO2 surface. The formation of PMC/CM-beta-CD was confirmed by fluorescence spectroscopy. The incident photon-to-current conversion efficiency (IPCE) of the PMCcontaining DSSC obtained 4.1% under 570-nm light irradiation, and the highest IPCE reached 11.1% by inclusion effect of CM-beta-CD. Similar, the fillfactor and the open-circuit voltage were improved by CM-beta -CD layer. The IPCE value of PMC/CM-beta-CD-containing DSSC was decreased by visible light treatment, and it was considered that decrease of IPCE values are attributed to the formation of PMC isomer. Therefore, we demonstrated the photovoltaic conversion and photoresponsivity of the DSSC by incorporating a noncarboxylated PMC with inclusion effect of CM-beta-CD layer. (C) 2016 Elsevier B.V. All rights reserved. | [Takeshita, Tatsuya; Umeda, Takao; Hara, Michihiro] Fukui Univ Technol, Dept Environm & Food Sci, 3-6-1 Gakuen, Fukui 9108505, Japan | Fukui University of Technology | Hara, M i˜A—’˜ŽÒjAFukui Univ Technol, Dept Environm & Food Sci, 3-6-1 Gakuen, Fukui 9108505, Japan. | hara@fukui-ut.ac.jp | Fukui University of Technology | Fukui University of Technology | This research was supported by Fukui University of Technology, Special Reserch Fund (2014-2016). | 22 | 10 | 10 | 0 | 64 | ELSEVIER SCIENCE SA | LAUSANNE | PO BOX 564, 1001 LAUSANNE, SWITZERLAND | 1010-6030 | 1873-2666 | J PHOTOCH PHOTOBIO A | J. Photochem. Photobiol. A-Chem. | JAN 15 | 2017 | 333 | 87 | 91 | 10.1016/j.jphotochem.2016.10.017 | http://dx.doi.org/10.1016/j.jphotochem.2016.10.017 | 5 | |||||||||
| Pugachev, AD; Rostovtseva, IA; Makarova, NI; Ievlev, MY; Dmitriev, VS; Ozhogin, IV; Tkachev, VV; Utenyshev, AN; Borodkina, IG; Metelitsa, AV; Aldoshin, SM; Minkin, VI; Luk'yanov, BS | Pugachev, A. D.; Rostovtseva, I. A.; Makarova, N. I.; Ievlev, M. Yu.; Dmitriev, V. S.; Ozhogin, I. V.; Tkachev, V. V.; Utenyshev, A. N.; Borodkina, I. G.; Metelitsa, A. V.; Aldoshin, S. M.; Minkin, V. I.; Luk'yanov, B. S. | Synthesis and study of new photochromic halogen-substituted spiropyrans of the indoline series | RUSSIAN CHEMICAL BULLETIN | spiropyran; photochromism; synthesis; CrystalExplorer; NMR; photovoltaics; DSSC | New indoline spiropyrans containing chlorine and bromine atoms in position 5 of the indoline moiety of the molecule as a substituent were synthesized and studied. The structures of the synthesized compounds were confirmed by NMR and IR spectroscopy. The molecular structure of the chlorine-substituted derivative was determined by X-ray diffraction analysis, and intermolecular interactions in the crystal were studied using the CrystalExplorer21.5 software package. The spectral kinetic studies revealed photochromic properties of novel spiropyrans in an acetonitrile solution. The photoelectrochemical characteristics of dye-sensitized solar cells (DSSC) made from the synthesized compounds before and after UV irradiation were studied in comparison. | [Pugachev, A. D.; Rostovtseva, I. A.; Makarova, N. I.; Dmitriev, V. S.; Ozhogin, I. V.; Borodkina, I. G.; Metelitsa, A. V.; Minkin, V. I.; Luk'yanov, B. S.] Southern Fed Univ, Res Inst Phys & Organ Chem, 194-2 Prosp Stachki, Rostov Na Donu 344090, Russia; [Ievlev, M. Yu.] Chuvash State Univ, 15 Moskovskii Prosp, Cheboksary 428015, Russia; [Tkachev, V. V.; Utenyshev, A. N.; Aldoshin, S. M.] Russian Acad Sci, Fed Res Ctr Problems Chem Phys & Med Chem, 1 Prosp Akad Semenova, Chernogolovka 142432, Moscow Region, Russia | Southern Federal University; Russian Academy of Sciences | Pugachev, AD i˜A—’˜ŽÒjASouthern Fed Univ, Res Inst Phys & Organ Chem, 194-2 Prosp Stachki, Rostov Na Donu 344090, Russia. | artem_d_pugachev@mail.ru | Minkin, Vladimir/C-9433-2013; Ozhogin, Ilya/L-8756-2016; Makarova, Nadezhda/B-1960-2017; Borodkina, Inna/R-6464-2016; Dmitriev, Vitaliy/JUF-0763-2023; Ievlev, Mikhail/E-7151-2016; Rostovtseva, Irina/LZF-0067-2025; Lukianov, Boris/H-3305-2013; Tkachev, Valery/AAC-1433-2021; Pugachev, Artem/F-9090-2017 | Ievlev, Mikhail/0000-0003-0741-2254; Dmitriev, Vitaliy/0000-0002-5519-4418 | Ministry of Science and Higher Education of the Russian Federation [FENW-2023-0020] | Ministry of Science and Higher Education of the Russian Federation | This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation in the framework of the state assignment in the area of scientific activities (Southern Federal University, registration No. FENW-2023-0020). The XRD study was performed in the framework of the state assignment (state registration No. AAAA-A19-119092390076-7). | 47 | 8 | 8 | 2 | 7 | SPRINGER | NEW YORK | ONE NEW YORK PLAZA, SUITE 4600, NEW YORK, NY, UNITED STATES | 1066-5285 | 1573-9171 | RUSS CHEM B+ | Russ. Chem. Bull. | NOV | 2023 | 72 | 11 | 2637 | 2648 | 10.1007/s11172-023-4068-7 | http://dx.doi.org/10.1007/s11172-023-4068-7 | 12 | |||||||
| Mavazzan, A; Mendhe, AC; Bayannavar, PK; Sankapal, BR; Kamble, RR; Madar, SF; Pasha, KMM; Bheemayya, L | Mavazzan, Ahmedraza; Mendhe, Avinash C.; Bayannavar, Praveen K.; Sankapal, Babasaheb R.; Kamble, Ravindra R.; Madar, Suresh F.; Pasha, K. M. Mussuvir; Bheemayya, Lokesh | Design of Metal Free Fluorescent Pyridine Dyes Anchored on Cadmium Sulfide Nanowires: Optical, Electrochemical and Photovoltaic Applications | JOURNAL OF FLUORESCENCE | Chalcone; DSSC; J-V study; EQE; CdS-NW study | HETEROCYCLIC CHALCONE ANALOGS; PHOTOCHROMIC BEHAVIOR; THIN-FILMS; TRIPHENYLAMINE; DERIVATIVES; EFFICIENCY; SPIROPYRAN; STABILITY; DSSC | Through a facile two-step synthetic procedure, three metal-free organic dyes having D-pi-A kind of structure, belonging to chalcone family have been designed, produced and anchored on one dimensional cadmium sulfide nanowires (1D CdS NWs) to serve as a light energy harvester through dye-sensitized solar cells (DSSC) assembly. In order to anchor dye on CdS NWs nano-network, solution chemistry has been used in an easy and effective manner. The sensitizing capability of synthesized materials has been evaluated using optical and electrochemical studies, density functional theory (DFT) simulations, and photovoltaic performances. In line with a detailed analysis of fabricated Dye sensitized solar cells containing T4PC a photovoltaic efficiency yields 4.35 times (0.487%) more than that of bare CdS NWs (0.112%), while the other devices having T3PC and T2PC have shown 3.0 (0.338%) and 2.40 (0.273%) times greater photovoltaic efficiencies, respectively under standard light illumination. The obtained results offer solid evidence in favour of boosting external quantum efficiency (EQE) and reflect good agreement with the optical studies. | [Mavazzan, Ahmedraza; Bayannavar, Praveen K.; Kamble, Ravindra R.; Madar, Suresh F.; Bheemayya, Lokesh] Karnatak Univ, Dept Studies Chem, Dharwad 580003, Karnataka, India; [Mendhe, Avinash C.] Hanyang Univ, Dept Civil & Environm Engn, ERICA, Ansan 15588, South Korea; [Mendhe, Avinash C.; Sankapal, Babasaheb R.] Visvesvaraya Natl Inst Technol, Dept Phys, Nanomat & Device Lab, South Ambazari Rd, Nagpur 440010, MS, India; [Pasha, K. M. Mussuvir] Karnatak Sci Coll, Dept Chem, Dharwad 580003, Karnataka, India | Karnatak University; Hanyang University; National Institute of Technology (NIT System); Visvesvaraya National Institute of Technology, Nagpur; Karnatak University | Kamble, RR i˜A—’˜ŽÒjAKarnatak Univ, Dept Studies Chem, Dharwad 580003, Karnataka, India. | ravichem@kud.ac.in | ; Sankapal, Babasaheb R./M-4759-2015; KAMBLE, RAVINDRA/AAH-7335-2021; Sankapal, Babasaheb/M-4759-2015 | Mendhe, Avinash/0000-0003-4513-1066; Sankapal, Babasaheb R./0000-0002-7464-9633; | We are grateful to DST, the Government of India, New Delhi and the University Scientific Instrumentation Centre (USIC), Karnatak University, Dharwad, India, for providing instrumentation facility viz., NMR, UV-Visible, Fluorescence, Powder X-ray and EDX-SE; Government of India, New Delhi; Government of Karnatak; University Grants Commission (UGC) | We are grateful to DST, the Government of India, New Delhi and the University Scientific Instrumentation Centre (USIC), Karnatak University, Dharwad, India, for providing instrumentation facility viz., NMR, UV-Visible, Fluorescence, Powder X-ray and EDX-SE; Government of India, New Delhi; Government of Karnatak; University Grants Commission (UGC)(University Grants Commission, India) | We are grateful to DST, the Government of India, New Delhi and the University Scientific Instrumentation Centre (USIC), Karnatak University, Dharwad, India, for providing instrumentation facility viz., NMR, UV-Visible, Fluorescence, Powder X-ray and EDX-SEM data under SAIF programme, and to Nanomaterials and Device Laboratory, Department of Physics, Visvesvaraya National Institute of Technology, Nagpur, India, for Solar cell Fabrication and analysis. One of the authors Ahmedraza Mavazzan (AM) thanks the Government of Karnatak for providing the GOKDOM Fellowship and University Grants Commission (UGC), Delhi for providing Maulana Azad National Fellowship (MANF). | 50 | 2 | 2 | 0 | 5 | SPRINGER/PLENUM PUBLISHERS | NEW YORK | 233 SPRING ST, NEW YORK, NY 10013 USA | 1053-0509 | 1573-4994 | J FLUORESC | J. Fluoresc. | SEP | 2024 | 34 | 5 | 2405 | 2414 | 10.1007/s10895-023-03457-z | http://dx.doi.org/10.1007/s10895-023-03457-z | OCT 2023 | 10 |
スピロピラン×DSSCの設計指針:CD包接と感光制御の最前線
概要
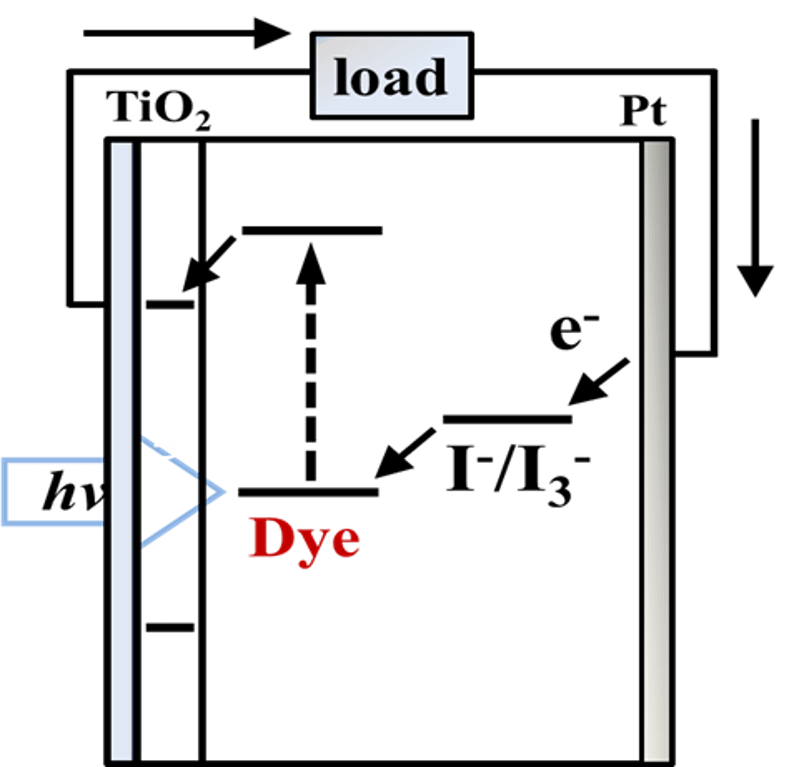
本セットは、スピロピラン(SP)派生色素を中心に、DSSC適用や基礎光応答特性を扱う3件を整理しました。1件はシクロデキストリン(CD)包接とSPの開環体(メロシアニン:MC)を活用したDSSC、1件はハロゲン置換SPの合成・光応答解析、1件は金属フリー蛍光色素×CdSナノワイヤの光電特性です。CD×SPは“書いて消せる”表示や光誘起相転移(VPTT)、**カスケードエネルギー移動(FRET)**によるゲーティング設計に有望です。
ここがポイント
- **CD包接×SP(MC)**で電極界面を設計:固定化と微視的環境制御で発色・応答を安定化。
- **アンカー基(カルボン酸等)**でTiO₂へ化学結合し、感光と電荷注入の両立を狙う。
- **置換設計(Cl/Br)**でSP↔MCの平衡・速度・吸収帯をチューニング。
- 小型発電・表示連携に向く半透明・可逆色変化。
- CD×SPの強み:デュアル架橋/VPTTによる相転移制御、FRETによるカスケード移動で設計自由度が高い。
論文別ハイライト
- Fabrication of a DSSC containing carboxylated SP-derived photomerocyanine with cyclodextrin(2017)
- 設計/材料:MC形SPと**CM-β-シクロデキストリン(CM-β-CD)**の包接層を用いたTiO₂電極。
- 機能/現象:SP→MC開環に伴う可視吸収増加を利用し、半透明発電と発色制御を両立。
- 数値的特長:PCEや時定数の詳細はN/A(包接・固定化の効果を中心に議論)。
- 用途像:**“書いて消せる”**窓型DSSC、ラベル・センサー一体化。
- Halogen-substituted indoline spiropyrans(2023)
- 設計/材料:Cl/Br置換SPの合成;結晶相互作用や分光・NMR評価。
- 機能/現象:SP↔MCの可逆光異性化と安定性・速度論の比較。
- 数値的特長:定量値はN/A(置換効果と結晶内相互作用の相関を提示)。
- 用途像:DSSC色素のプリカーサ、色調スイッチの候補。
- Metal-free fluorescent pyridine dyes on CdS nanowires(2024)
- 設計/材料:金属フリー蛍光色素+CdSナノワイヤネットワーク。
- 機能/現象:J–V/EQE評価による光電・電気化学特性の検証。
- 数値的特長:具体値はN/A(手法・設計指針を示す)。
- 用途像:発光・発電ハイブリッド、共感受設計の参考。
用語ミニ解説
- スピロピラン(SP)/メロシアニン(MC):UVなどでSP→MCに開環し可視吸収が増加する可逆系。
- シクロデキストリン(CD, α/β/γ):疎水性空洞により包接複合体を形成、色素の固定・保護に有用。
- FRET(カスケードエネルギー移動):ドナー→アクセプターへ無輻射移動;SP→色素の受け渡し設計に活用。
- VPTT(体積相転移温度):温度でゲル体積が急変;光→熱連成で光誘起相転移を実装可能。
- アンカー基:カルボン酸等,TiO₂表面への化学結合に用いる基。
想定アプリケーション
- 半透明DSSC+可逆表示(窓・ラベル・屋内IoT表示)
- BIPVでの意匠・遮光制御
- 光応答ゲルバルブ/センサー(CD×SP×VPTT)
- 教育展示:光異性化・エネルギー移動の可視化
関連キーワード
DSSC、スピロピラン、メロシアニン、シクロデキストリン、包接複合体、FRET、VPTT、TiO₂電極、ハロゲン置換、CdSナノワイヤ
Design Rules for Spiropyran-Enabled DSSCs: CD Inclusion and Light-Driven Control
Overview
This 3-paper set covers (i) a cyclodextrin (CD)-assisted DSSC employing the merocyanine (MC) form of spiropyran, (ii) halogen-substituted indoline spiropyrans and their photochromism, and (iii) metal-free fluorescent dyes on CdS nanowires with J–V/EQE characterization. CD×SP architectures are promising for write-erase displays, VPTT-mediated phase switching, and cascade FRET gating in semi-transparent PV.
Why it matters / Key points
- CD inclusion with MC-SP stabilizes interfacial microenvironments and improves fixation on TiO₂.
- Carboxylate anchoring secures dyes to TiO₂ while enabling light-driven color change.
- Halogen substitution (Cl/Br) tunes SP↔MC equilibria and kinetics.
- Reversible chromism aligns with aesthetic, semi-transparent PV use cases.
- CD×SP advantages: dual cross-linking/VPTT control and FRET-based cascade energy transfer.
Highlights by study
- DSSC with carboxylated SP-derived merocyanine and cyclodextrin(2017)
- Design: MC-SP with CM-β-CD inclusion on TiO₂.
- Function: Visible-absorption increase upon SP→MC switching; semi-transparent PV + color control.
- Metrics: N/A (focus on inclusion/fixation effects).
- Use: Write-erase window-PV, smart labels/sensors.
- Halogenated indoline spiropyrans(2023)
- Design: Cl/Br-SP synthesis; crystal/kinetic/spectral analyses.
- Function: Reversible SP↔MC photochromism and stability trends.
- Metrics: N/A (substitution–property correlations).
- Use: Precursors for DSSC dyes; color-switchable tags.
- Metal-free fluorescent pyridine dyes on CdS NWs(2024)
- Design: Metal-free dyes on CdS nanowire networks.
- Function: J–V/EQE-based opto-electrochemical assessment.
- Metrics: N/A (method/architecture pointers).
- Use: Hybrid emission/harvesting; co-sensitization references.
Mini-glossary
- SP/MC: UV-induced SP→MC ring opening with enhanced visible absorption (reversible).
- Cyclodextrin (CD): Host for inclusion complexes; aids dye fixation/protection.
- FRET: Non-radiative cascade energy transfer from donor to acceptor.
- VPTT: Volume phase transition temperature; enables light-induced phase switching via photothermal coupling.
- Anchoring group: Carboxylate etc., for TiO₂ chemisorption.
Potential applications
- Semi-transparent DSSCs with reversible display (windows/labels/IoT)
- BIPV façades with adaptive tinting
- Photoresponsive gel valves/sensors (CD×SP×VPTT)
- Education/demos on photoisomerization & energy transfer
Suggested tags
DSSC, spiropyran, merocyanine, cyclodextrin, inclusion complex, FRET, VPTT, TiO2 electrode, halogen substitution, CdS nanowire
参考文献(入力順)
- Takeshita, T.; Umeda, T.; Hara, M.. Fabrication of a dye-sensitized solar cell containing carboxylated spiropyran-derived photomerocyanine with cyclodextrin. Journal of Photochemistry and Photobiology A: Chemistry 2017, 333(N/A), 87–91. https://doi.org/10.1016/j.jphotochem.2016.10.017
- Pugachev, A.; Rostovtseva, I.; Makarova, N.; Ievlev, M.; Dmitriev, V.; Ozhogin, I.; et al. Synthesis and study of new photochromic halogen-substituted spiropyrans of the indoline series. Russian Chemical Bulletin 2023, 72(11), 2637–2648. https://doi.org/10.1007/s11172-023-4068-7
- Mavazzan, A.; Mendhe, A.; Bayannavar, P.; Sankapal, B.; Kamble, R.; Madar, S.; et al. Design of Metal Free Fluorescent Pyridine Dyes Anchored on Cadmium Nanowires: Optical, Electrochemical and Photovoltaic Applications. Journal of Fluorescence 2024, 34(5), 2405–2414. https://doi.org/10.1007/s10895-023-03457-z