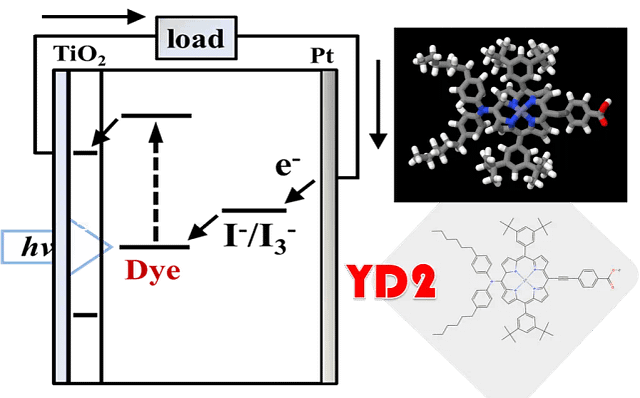
TD-DFT and YD2
| Authors | Book Authors | Book Editors | Book Group Authors | Author Full Names | Book Author Full Names | Group Authors | Article Title | Source Title | Book Series Title | Book Series Subtitle | Language | Document Type | Conference Title | Conference Date | Conference Location | Conference Sponsor | Conference Host | Author Keywords | Keywords Plus | Abstract | Addresses | Affiliations | Reprint Addresses | Email Addresses | Researcher Ids | ORCIDs | Funding Orgs | Funding Name Preferred | Funding Text | Cited References | Cited Reference Count | Times Cited, WoS Core | Times Cited, All Databases | 180 Day Usage Count | Since 2013 Usage Count | Publisher | Publisher City | Publisher Address | ISSN | eISSN | ISBN | Journal Abbreviation | Journal ISO Abbreviation | Publication Date | Publication Year | Volume | Issue | Part Number | Supplement | Special Issue | Meeting Abstract | Start Page | End Page | Article Number | DOI | DOI Link | Book DOI | Early Access Date | Number of Pages | WoS Categories | Web of Science Index | Research Areas | IDS Number | Pubmed Id | Open Access Designations | Highly Cited Status | Hot Paper Status | Date of Export | UT (Unique WOS ID) | Web of Science Record |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee, GH; Kim, YS | Lee, Geon Hyeong; Kim, Young Sik | Theoretical study of novel porphyrin-based dye for efficient dye-sensitized solar cell | MOLECULAR CRYSTALS AND LIQUID CRYSTALS | English | Article | Porphyrin; dye-sensitized solar cell; DSSC; DFT; TDDFT | ORGANIC-DYES; ELECTROLYTE; ACCEPTOR; COMPLEX | In this study, novel porphyrin-based dye sensitizer with diphenylamine (DPA) as the electron donating group, isoindigo (II) as the electron withdrawing group, and carboxylic acid (CA) with benzene as the anchoring part (YD2-II-CA) were theoretically investigated in comparison with the recent best dye (YD2-o-C8). Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were used to gain insight into the factors responsible for photovoltaic performance. Due to the substitution of the electron withdrawing unit, the absorption spectra of YD2-II-CA was more red-shifted and broader than the spectrum of the YD2-o-C8. The results suggest that this novel porphyrin-based dye would have good photovoltaic properties in dye-sensitized solar cells (DSSCs) | [Lee, Geon Hyeong; Kim, Young Sik] Hongik Univ, Dept Informat Display, Seoul 121791, South Korea; [Kim, Young Sik] Hongik Univ, Dept Sci, Seoul, South Korea | Hongik University; Hongik University | Kim, YS i˜A—’˜ŽÒjAHongik Univ, Dept Informat Display, Seoul 121791, South Korea.;Kim, YS i˜A—’˜ŽÒjAHongik Univ, Dept Sci, Seoul, South Korea. | youngkim@hongik.ac.kr | Kim, Young-Sik/AFC-8873-2022 | Basic Science Research Program through the National Research Foundation of Korea (NRF) - Ministry of Education, Science and Technology [2010-0021668] | Basic Science Research Program through the National Research Foundation of Korea (NRF) - Ministry of Education, Science and Technology | This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021668). | 15 | 1 | 2 | 1 | 46 | TAYLOR & FRANCIS LTD | ABINGDON | 2-4 PARK SQUARE, MILTON PARK, ABINGDON OR14 4RN, OXON, ENGLAND | 1542-1406 | 1563-5287 | MOL CRYST LIQ CRYST | Mol. Cryst. Liquid Cryst. | 2017 | 645 | 1 | 168 | 174 | 10.1080/15421406.2016.1277636 | http://dx.doi.org/10.1080/15421406.2016.1277636 | 7 | Chemistry, Multidisciplinary; Crystallography; Materials Science, Multidisciplinary | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry; Crystallography; Materials Science | EU7DH | 2025-09-12 | WOS:000401194700022 | View Full Record in Web of Science | |||||||||||||||||||||||||||
| Shalabi, AS; El Mahdy, AM; Taha, HO; Soliman, KA | Shalabi, A. S.; El Mahdy, A. M.; Taha, H. O.; Soliman, K. A. | The effects of macrocycle and anchoring group replacements on the performance of porphyrin based sensitizer: DFT and TD-DFT study | JOURNAL OF PHYSICS AND CHEMISTRY OF SOLIDS | English | Article | Porphyrins; Phthalocyanines; Anchoring groups; DSSCs; TD-DFT | PUSH-PULL PORPHYRINS; SOLAR-CELLS; ELECTRONIC-PROPERTIES; MOLECULAR DESIGN; AB-INITIO; DYE; PHTHALOCYANINES; ENERGIES; IMPROVEMENT; EFFICIENCY | Density functional theory and time dependent-density functional theory calculations have been carried out in an attempt to design new phthalocycanine based sensitizers that could be expected to improve the performance of the porphyrin based sensitizer YD2-o-C8. This was done through replacing the porphyrin macrocycle and carboxylic acid anchoring group of YD2-o-C8 by phthalocyanine macrocycle and cyanoacrylic acid anchoring group, respectively. The performances of the suggested cells could be expected to improve the efficiency of the reference dye YD2-o-C8 with Ti38O76, (TiO2)(60), SiC, and SrTiO3 semiconductors. Macrocycle replacement assists in promoting the efficiency in the red shoulder of the spectrum more effectively than that of the anchoring group. The effects of the former structural modifications on cell performance are confirmed in terms of frontier molecular orbitals, energy gaps, semiconductor valence and conduction band edges, density of states, molecular electrostatic potentials, non linear optical performances, reorganization energies, UV-vis absorption and emission, life times of excited states, light harvesting efficiency, injection efficiency, charge collection, and free energy of regeneration. (C) 2014 Elsevier Ltd. All rights reserved. | [Shalabi, A. S.; Soliman, K. A.] Benha Univ, Fac Sci, Dept Chem, Banha, Egypt; [El Mahdy, A. M.; Taha, H. O.] Ain Shams Univ, Fac Educ, Dept Phys, Cairo, Egypt | Egyptian Knowledge Bank (EKB); Benha University; Egyptian Knowledge Bank (EKB); Ain Shams University | Shalabi, AS i˜A—’˜ŽÒjABenha Univ, Fac Sci, Dept Chem, POB 13518, Banha, Egypt. | asshalabi@hotmail.com | Osman, Hayam/MHQ-0787-2025; soliman, kamal/HHM-5647-2022 | El mahdy, Atef/0000-0002-8292-4172; Taha, Hayam Osman/0000-0001-8222-2958; | 61 | 39 | 42 | 0 | 66 | PERGAMON-ELSEVIER SCIENCE LTD | OXFORD | THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLAND | 0022-3697 | 1879-2553 | J PHYS CHEM SOLIDS | J. Phys. Chem. Solids | JAN | 2015 | 76 | 22 | 33 | 10.1016/j.jpcs.2014.08.002 | http://dx.doi.org/10.1016/j.jpcs.2014.08.002 | 12 | Chemistry, Multidisciplinary; Physics, Condensed Matter | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry; Physics | AT8LB | 2025-09-12 | WOS:000345183700004 | View Full Record in Web of Science | |||||||||||||||||||||||||||||
| Kang, GJ; Song, C; Ren, XF | Kang, Guo-Jun; Song, Chao; Ren, Xue-Feng | Charge Transfer Enhancement in the D-ƒÎ-A Type Porphyrin Dyes: A Density Functional Theory (DFT) and Time-Dependent Density Functional Theory (TD-DFT) Study | MOLECULES | English | Article | DSSCs; charge transfer; DFT; porphyrin | SENSITIZED SOLAR-CELLS; ORGANIC-DYES; TIO2; PERFORMANCE; ABSORPTION; EFFICIENCY; DESIGN; DERIVATIVES; CONVERSION; DSSCS | The electronic geometries and optical properties of two D-pi-A type zinc porphyrin dyes (NCH3-YD2 and TPhe-YD) were systematically investigated by density functional theory (DFT) and time-dependent density functional theory (TD-DFT) to reveal the origin of significantly altered charge transfer enhancement by changing the electron donor of the famous porphyrin-based sensitizer YD2-o-C8. The molecular geometries and photophysical properties of dyes before and after binding to the TiO2 cluster were fully investigated. From the analyses of natural bond orbital (NBO), extended charge decomposition analysis (ECDA), and electron density variations (Delta rho) between the excited state and ground state, it was found that the introduction of N(CH3)(2) and 1,1,2-triphenylethene groups enhanced the intramolecular charge-transfer (ICT) character compared to YD2-o-C8. The absorption wavelength and transition possess character were significantly influenced by N(CH3)(2) and 1,1,2-triphenylethene groups. NCH3-YD2 with N(CH3)(2) groups in the donor part is an effective way to improve the interactions between the dyes and TiO2 surface, light having efficiency (LHE), and free energy change (Delta G(inject)), which is expected to be an efficient dye for use in dye-sensitized solar cells (DSSCs). | [Kang, Guo-Jun; Song, Chao; Ren, Xue-Feng] China Univ Min & Technol, Sch Chem Engn & Technol, Low Carbon Energy Inst, Xuzhou 221008, Peoples R China | China University of Mining & Technology | Ren, XF i˜A—’˜ŽÒjAChina Univ Min & Technol, Sch Chem Engn & Technol, Low Carbon Energy Inst, Xuzhou 221008, Peoples R China. | gjkang@cumt.edu.cn; schaocumt@126.com; renxf@cumt.edu.cn | song, chao/HTO-8527-2023 | Fundamental Research Funds for the Central Universities [2013QNA14]; Priority Academic Program Development of Jiangsu Higher Education Institutions | Fundamental Research Funds for the Central Universities(Fundamental Research Funds for the Central Universities); Priority Academic Program Development of Jiangsu Higher Education Institutions | Financial supports were received from the Fundamental Research Funds for the Central Universities (Nos. 2013QNA14). A Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions is acknowledged. The authors are grateful to the High Performance Computing Center of China University of Mining and Technology for the award of CPU hours to accomplish this work. | 45 | 10 | 12 | 2 | 54 | MDPI | BASEL | MDPI AG, Grosspeteranlage 5, CH-4052 BASEL, SWITZERLAND | 1420-3049 | MOLECULES | Molecules | DEC | 2016 | 21 | 12 | 1618 | 10.3390/molecules21121618 | http://dx.doi.org/10.3390/molecules21121618 | 12 | Biochemistry & Molecular Biology; Chemistry, Multidisciplinary | Science Citation Index Expanded (SCI-EXPANDED) | Biochemistry & Molecular Biology; Chemistry | EI0CQ | 27897999 | gold, Green Published, Green Submitted | 2025-09-12 | WOS:000392140100012 | View Full Record in Web of Science | ||||||||||||||||||||||||||
| Xie, M; Wang, J; Ren, J; Hao, L; Bai, FQ; Pan, QJ; Zhang, HX | Xie, Miao; Wang, Jian; Ren, Jie; Hao, Li; Bai, Fu-Quan; Pan, Qing-Jiang; Zhang, Hong-Xing | Theoretical study on a high-efficient porphyrin-sensitizer in a local electric field: How does the local electric field affects the performance of dye-sensitized solar cells? | ORGANIC ELECTRONICS | English | Article | Dye-sensitized solar cell; Porphyrin sensitizers; TD-DFT; Absorption spectrum; Local electric field | DENSITY-FUNCTIONAL THEORY; PHOTOINDUCED ABSORPTION; EXCITATION-ENERGIES; ORGANIC-DYES; TIME; DESIGN; SPECTROSCOPY; TECHNOLOGY; MOLECULES; SURFACE | The local electric field formed between dye sensitizers and semiconductor interface is one of key factors to determine the overall performance of dye-sensitized solar cells (DSSCs). Herein, a strategy has been proposed to explore the influence of the local electric field on the functionality of DSSCs of YD2-O-C8 dye via calculating the relevant properties in various electric field strengths. The YD2-O-C8 dye has been systemically studied with density functional theory (DFT) and time-dependent DFT (TD-DFT) for its electronic structure and optical properties in tetrahydrofuran (THF) solution. The absorption spectra are gradually narrowing and blue-shifting while increasing the electric field strength. Two key parameters of the light harvesting efficiency (LHE) and the TiO2 conduction band shift (Delta E-cb) have been examined for the YD2-O-C8 sensitized TiO2 system. It is found that it is of great importance to reduce the charge accumulation on the TiO2 film, which lowers the electric field strength and shows the best performance of DSSCs. This study is expected to deepen our understanding of the function of local electric field and the operational principles of the DSSCs for further optimization. (C) 2015 Elsevier B.V. All rights reserved. | [Xie, Miao; Wang, Jian; Ren, Jie; Hao, Li; Bai, Fu-Quan; Zhang, Hong-Xing] Jilin Univ, Inst Theoret Chem, Changchun 130023, Peoples R China; [Pan, Qing-Jiang] Heilongjiang Univ, Sch Chem & Mat Sci, Educ Minist, Key Lab Funct Inorgan Mat Chem, Harbin 150080, Peoples R China | Jilin University; Heilongjiang University | Bai, FQ i˜A—’˜ŽÒjAJilin Univ, Inst Theoret Chem, Changchun 130023, Peoples R China. | baifq@jlu.edu.cn; panqjitc@163.com; zhanghx@mail.jlu.edu.cn | ; wang, jian/GVS-0711-2022; Bai, Fu-Quan/ACG-2583-2022 | Wang, Abbott/0000-0003-4670-7741; Xie, Miao/0000-0002-9797-1449; Bai, Fu-Quan/0000-0001-9398-1407 | Natural Science Foundation of China [21173096, 21203071]; State Key Development Program for Basic Research of China [2013CB834801]; China Postdoctoral Science Foundation [2013M541288] | Natural Science Foundation of China(National Natural Science Foundation of China (NSFC)); State Key Development Program for Basic Research of China(State Key Development Program for Basic Research of China); China Postdoctoral Science Foundation(China Postdoctoral Science Foundation) | This work was supported by the Natural Science Foundation of China (Grant Nos. 21173096 and 21203071) and the State Key Development Program for Basic Research of China (Grant No. 2013CB834801) and China Postdoctoral Science Foundation (Grant No. 2013M541288). | 42 | 19 | 21 | 2 | 123 | ELSEVIER | AMSTERDAM | RADARWEG 29, 1043 NX AMSTERDAM, NETHERLANDS | 1566-1199 | 1878-5530 | ORG ELECTRON | Org. Electron. | NOV | 2015 | 26 | 164 | 175 | 10.1016/j.orgel.2015.07.045 | http://dx.doi.org/10.1016/j.orgel.2015.07.045 | 12 | Materials Science, Multidisciplinary; Physics, Applied | Science Citation Index Expanded (SCI-EXPANDED) | Materials Science; Physics | CR3IX | 2025-09-12 | WOS:000361226900026 | View Full Record in Web of Science | ||||||||||||||||||||||||||
| Demirci, YC; ?akar, S; Sevim, AM; G?l, A; ?zacar, M | Demirci, Yigit Can; Cakar, Soner; Sevim, Altug Mert; Gul, Ahmet; Ozacar, Mahmut | DSSCs based on unsymmetrical A3B type Zn(II) and TiO(IV) naphthalenephthalocyanine/porphyrin cocktail dyes: A potential alternative for ruthenium based sensitizers | JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY | English | Article | Phthalocyanines; Porphyrins; Cocktail dyes; Metal complexes dyes; Dye-sensitized solar cells (DSSC); Solar energy; Density-functional theory | ZINC PHTHALOCYANINE SENSITIZERS; SOLAR-CELLS; CO-SENSITIZATION; ORGANIC-DYE; PERFORMANCE; COMPLEX | To increase the efficiency of dye-sensitized solar cells (DSSCs), there is still a need for alternative dyes that can absorb a broad spectrum of sunlight and maintain high conversion efficiencies. In order to meet these requirements, a cocktail dye made up of novel phthalocyanines together with a porphyrin have been designed. The novel unsymmetrical phthalocyanines are two ferrocenylphenoxy-naphthalenephthalocyanine (FPNPc) with Zn (ZnFPNPc) or TiO (TiOFPNPc) at the inner core and 4-ferrocenylphenoxy and 4-carboxybenzo substituents at peripheral positions. In order to increase their UV-Vis absorption capacity, cocktails have been prepared with the synthesized new dyes (ZnFPNPc or TiOFPNPc) and YD2 porphyrin dye. The full characterization of novel synthesized dyes has been performed by UV-Vis absorption, FTIR, H1-NMR, MALDI-TOF, electrochemical, TD-DFT techniques. In this work we have prepared DSSCs based on YD2 and phthalocyanine dye molecule with different cocktail ratio (YD2 + ZnFPNPc or YD2 + TiOFPNPc ratios: 1:1, 1:2, 2:1, 1:3, 3:1, 1:4 and 4:1). The best solar cell performance has been performed in the cases of YD2 + ZnFPNPc (3:1) (9.96%) and YD2 + TiOFPNPc (3:1) (10.72%) cocktail dyes. Due to the high efficiency values obtained, these new cocktail dyes are expected to be a good alternative to the ruthenium-based complex dyes used in DSSCs and pave the way for potential alternative dyes. | [Demirci, Yigit Can; Ozacar, Mahmut] Sakarya Univ, Fac Sci, Dept Chem, TR-54187 Sakarya, Turkiye; [Cakar, Soner] Zonguldak Bulent Ecevit Univ, Dept Chem, TR-67100 Zonguldak, Turkiye; [Cakar, Soner; Ozacar, Mahmut] Sakarya Univ, Biomat Energy Photocatalysis Enzyme Technol Nano &, TR-54187 Sakarya, Turkiye; [Sevim, Altug Mert; Gul, Ahmet] Istanbul Tech Univ, Dept Chem, TR-34469 Istanbul, Turkiye | Sakarya University; Zonguldak Bulent Ecevit University; Sakarya University; Istanbul Technical University | ?zacar, M i˜A—’˜ŽÒjASakarya Univ, Fac Sci, Dept Chem, TR-54187 Sakarya, Turkiye. | mozacar@sakarya.edu.tr | ?zacar, Mahmut/AAF-9122-2020; GUL, AHMET/LXW-1956-2024; Sevim, Altu?/Y-4197-2018; ?akar, Soner/AAH-1477-2020 | Ozacar, Mahmut/0000-0002-1783-7275; | Scientific and Technological Research Council of Turkey (TUBITAK) [119Z082]; Scientific Research Projects Commission of Zonguldak Bulent Ecevit University [2021-72118496-04]; Turkish Academy of Sciences (TUBA) | Scientific and Technological Research Council of Turkey (TUBITAK)(Turkiye Bilimsel ve Teknolojik Arastirma Kurumu (TUBITAK)); Scientific Research Projects Commission of Zonguldak Bulent Ecevit University; Turkish Academy of Sciences (TUBA)(Turkish Academy of Sciences) | This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) (Project number:119Z082) and Scientific Research Projects Commission of Zonguldak Bulent Ecevit University (Project Number: 2021-72118496-04) . A.G. and M. O. thank Turkish Academy of Sciences (TUBA) for partial support. | 45 | 12 | 12 | 1 | 22 | ELSEVIER SCIENCE SA | LAUSANNE | PO BOX 564, 1001 LAUSANNE, SWITZERLAND | 1010-6030 | 1873-2666 | J PHOTOCH PHOTOBIO A | J. Photochem. Photobiol. A-Chem. | JUN 1 | 2023 | 440 | 114642 | 10.1016/j.jphotochem.2023.114642 | http://dx.doi.org/10.1016/j.jphotochem.2023.114642 | FEB 2023 | 10 | Chemistry, Physical | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry | N9HU3 | 2025-09-12 | WOS:001040046800001 | View Full Record in Web of Science | ||||||||||||||||||||||||||
| Li, XY; Zhang, CR; Yuan, LH; Zhang, ML; Chen, YH; Liu, ZJ | Li, Xing-Yu; Zhang, Cai-Rong; Yuan, Li-Hua; Zhang, Mei-Ling; Chen, Yu-Hong; Liu, Zi-Jiang | A comparative study of porphyrin dye sensitizers YD2-o-C8, SM315 and SM371 for solar cells: the electronic structures and excitation-related properties | EUROPEAN PHYSICAL JOURNAL D | English | Article | DENSITY-FUNCTIONAL THEORY; ORGANIC-DYES; ABSORPTION PROPERTIES; MOLECULAR DESIGN; DFT/TD-DFT; EFFICIENCY; TIO2; COMPLEXES; ENERGY; DERIVATIVES | Understanding the electronic structures and excitation properties of dye sensitizers has significant importance to improve the photon-energy conversion efficiency (PCE) of dye-sensitized solar cells (DSSCs). Here, based upon the results calculated using density functional theory, the electronic structures and excitation related properties of porphyrin dye sensitizers YD2-o-C8, SM315, and SM371 were analyzed. It was found that the similar electronic structures of YD2-o-C8 and SM371 result in similar absorption spectra, excitation, and free energy variation for electron injection (EI) and dye regeneration. However, since the electronic structure of the benzothiadiazole unit is well-coupled to that of the porphyrin ring, introducing benzothiadiazole into porphyrin dyes generates a decrease in the lowest unoccupied molecular orbital energy, red-shift and splitting of absorption bands. Meanwhile, remarkably it increases the transferred charges of excitation, which is responsible for the superior short-circuit current density of SM315 sensitized DSSCs. Furthermore, the transition configurations and molecular orbitals indicate the diarylamine group acts as an electronic donor, and the different EI modes with different timescales coexist in excited states due to the multi-configurations of transition. The results of structure-property relationships are favorable to develop novel dye sensitizers for DSSCs. | [Li, Xing-Yu; Zhang, Cai-Rong; Chen, Yu-Hong] Lanzhou Univ Technol, State Key Lab Adv Proc & Recycling Non ferrous Me, Lanzhou 730050, Gansu, Peoples R China; [Li, Xing-Yu; Zhang, Cai-Rong; Yuan, Li-Hua; Zhang, Mei-Ling; Chen, Yu-Hong] Lanzhou Univ Technol, Dept Appl Phys, Lanzhou 730050, Gansu, Peoples R China; [Liu, Zi-Jiang] Lanzhou City Univ, Dept Phys, Lanzhou 730070, Peoples R China | Lanzhou University of Technology; Lanzhou University of Technology; Lanzhou City University | Zhang, CR i˜A—’˜ŽÒjALanzhou Univ Technol, State Key Lab Adv Proc & Recycling Non ferrous Me, Lanzhou 730050, Gansu, Peoples R China.;Zhang, CR i˜A—’˜ŽÒjALanzhou Univ Technol, Dept Appl Phys, Lanzhou 730050, Gansu, Peoples R China. | zhcrxy@lut.cn | ZHANG, CAI-RONG/E-3126-2013; Liu, Zi-Jiang/N-7164-2013; CHEN, YuHong/J-5863-2013; Li, Xingyu/AHE-0001-2022; Liu, Zi-Jiang/L-1895-2019 | Liu, Zi-Jiang/0000-0002-0880-8149; | National Natural Science Foundation of China [11164016] | National Natural Science Foundation of China(National Natural Science Foundation of China (NSFC)) | This work was supported by the National Natural Science Foundation of China (Grant No. 11164016). The authors were grateful to the high-performance computing platform of Lanzhou University of Technology. The authors were also appreciative of the National Supercomputing Center in Shenzhen. Xing-Yu Li thanks Li-Heng Han for assistance. | 74 | 13 | 14 | 0 | 31 | SPRINGER | NEW YORK | ONE NEW YORK PLAZA, SUITE 4600, NEW YORK, NY, UNITED STATES | 1434-6060 | 1434-6079 | EUR PHYS J D | Eur. Phys. J. D | OCT 18 | 2016 | 70 | 10 | 211 | 10.1140/epjd/e2016-70071-3 | http://dx.doi.org/10.1140/epjd/e2016-70071-3 | 10 | Optics; Physics, Atomic, Molecular & Chemical | Science Citation Index Expanded (SCI-EXPANDED) | Optics; Physics | EQ7KA | 2025-09-12 | WOS:000398261700001 | View Full Record in Web of Science | |||||||||||||||||||||||||||
| Yasin, A; Jose, R; Yusoff, MM | Yasin, Amina; Jose, Rajan; Yusoff, Mashitah M. | Predicting larger absorption cross-section in porphyrin dyes using DFT calculations | JOURNAL OF PORPHYRINS AND PHTHALOCYANINES | English | Article | Zn(II)porhyrin dyes; DSSCs; DFT; TD-DFT; electronic properties; absorption cross-ction | SENSITIZED SOLAR-CELLS; DENSITY-FUNCTIONAL THEORY; CONJUGATED ORGANIC-DYES; ADSORBING GROUPS; COUMARIN DYE; EFFICIENCY; TIO2; COMPLEXES; THIOPHENE; DESIGN | Porphyrin macrocycles play an important role in designing of fluorophores with superior light harvesting properties similar to that of antennas in biological systems. In this paper, new Zn(II) porphyrin dyes were investigated to improve the performance of the YD2-o-C8 using density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. Effects of various substituted and anchoring groups on basic porphine and Zn(II) porphyrin derivatives were systematically studied at the B3LYP/LanL2DZ level. The absorption spectra of Zn(II) porphyrin derivatives bearing one, two and four anchoring groups in the meso-positions were also studied. The calculations showed that a molecule [5, 10, 15, 20-(4-carboxyphenylethynyl)porphyrinato]Zn(II) have large absorption cross-ection than available in the existing porphyrin dyes. The results of these calculations would open up enormous possibilities to develop porphyrin dyes characterized by high absorption cross-section for various light harvesting applications. | [Yasin, Amina; Jose, Rajan; Yusoff, Mashitah M.] Univ Malaysia Pahang, Fac Ind Sci & Technol, Kuantan 26300, Pahang, Malaysia | Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA) | Jose, R i˜A—’˜ŽÒjAUniv Malaysia Pahang, Fac Ind Sci & Technol, Kuantan 26300, Pahang, Malaysia. | rjose@ump.edu.my; mashitah@ump.edu.my | Jose, Rajan/C-9944-2009; yasin, amina/HKE-7683-2023; Yusoff, Mashitah/J-5975-2012 | Yusoff, Mashitah/0000-0003-4118-5307; Yasin, Amina/0009-0000-0193-2166; Jose, Rajan/0000-0003-4540-321X | Research & Innovation Department of Universiti Malaysia Pahang (UMP) [RDU 150328] | Research & Innovation Department of Universiti Malaysia Pahang (UMP) | This research is supported by the Research & Innovation Department of Universiti Malaysia Pahang (UMP) through the grant entitled Synthesis of Porphyrins with Tailored Optical Properties (RDU 150328). | 62 | 8 | 8 | 3 | 31 | WORLD SCI PUBL CO INC | HACKENSACK | 27 WARREN ST, STE 401-402, HACKENSACK, NJ 07601 USA | 1088-4246 | 1099-1409 | J PORPHYR PHTHALOCYA | J. Porphyr. Phthalocyanines | DEC | 2015 | 19 | 12 | 1270 | 1278 | 10.1142/S1088424615501138 | http://dx.doi.org/10.1142/S1088424615501138 | 9 | Chemistry, Multidisciplinary | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry | DE0EO | 2025-09-12 | WOS:000370297900008 | View Full Record in Web of Science | |||||||||||||||||||||||||
| Gao, QQ; Yuan, QT; Song, XF; Yu, YM; Guo, J | Gao Qian-Qian; Yuan Qing-Tang; Song Xu-Feng; Yu Yan-Min; Guo Jing | Theoretical Study on Regulation of Photoelectric Properties of Zinc Porphyrin Dyes with Heterocyclopentadiene as ƒÎ-Bridge | CHINESE JOURNAL OF INORGANIC CHEMISTRY | Chinese | Article | porphyrin; dye; heterocyclopentadien; pi-bridge; density functional theory | SENSITIZED SOLAR-CELLS; DESIGN; THERMOCHEMISTRY; PERFORMANCE | To explore the regulation of photoelectric properties of zinc porphyrin dyes with heterocyclopentadiene as pi-bridge, six new zinc porphyrin dyes were designed by introducing heterocyclopentadiene with different heteroatoms as the pi-bridge based on the reference dye YD2-o-C8. The frontier molecular orbital energy levels, absorption spectra, and the hole-electron separation characteristics of the designed dyes were investigated using the density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods. The results show that compared with the reference dye YD2-o-C8, the introduction of heterocyclopentadiene in pi-bridge can improve the photoelectric performance of dyes. The photoelectric properties of porphyrin dyes can be regulated by changing heteroatoms. Further analysis on the relationship between the properties of heterocyclopentadienes and the photoelectric performance of the porphyrin dyes shows that the lowest unoccupied molecular orbital energy level of heterocyclopentadienes has a good linear relationship with the photoelectric properties of the designed porphyrin dyes. The stronger electron receiving ability of heterocyclopentadiene can lead to the better performance of the porphyrin dye. The silicon-heterocyclopentadiene is of the strongest electron receiving ability, and the corresponding porphyrin dye has the widest absorption spectra and the strongest intramolecular charge transfer ability. | [Gao Qian-Qian; Yuan Qing-Tang; Song Xu-Feng; Yu Yan-Min; Guo Jing] Beijing Univ Technol, Dept Environm Chem Engn, Beijing Key Lab Green Catalysis & Separat, Beijing 100124, Peoples R China | Beijing University of Technology | Yu, YM i˜A—’˜ŽÒjABeijing Univ Technol, Dept Environm Chem Engn, Beijing Key Lab Green Catalysis & Separat, Beijing 100124, Peoples R China. | ymyu@bjut.edu.cn | Gao, Qianqian/AAW-1689-2020 | 41 | 2 | 2 | 0 | 23 | CHINESE CHEMICAL SOC | BEIJING | C/O DEPT INT AFFAIRS, SECRETARY OF CHEM SOC, PO BOX 2709, BEIJING 100080, PEOPLES R CHINA | 1001-4861 | CHINESE J INORG CHEM | Chin. J. Inorg. Chem. | FEB | 2022 | 38 | 2 | 295 | 303 | 10.11862/CJIC.2022.018 | http://dx.doi.org/10.11862/CJIC.2022.018 | 9 | Chemistry, Inorganic & Nuclear | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry | 1B0MK | 2025-09-12 | WOS:000792139200012 | View Full Record in Web of Science | ||||||||||||||||||||||||||||||
| Arslan, N; Yildirir, SN; Sevim, AM; ?akar, S; ?zacar, M; G?l, A | Arslan, Nuray; Yildirir, Seyma Nur; Sevim, Altug Mert; Cakar, Soner; Ozacar, Mahmut; Gul, Ahmet | Sulfur-bridged oxotitanium phthalocyanine- and porphyrin-based cocktail dyes as sensitizers for improved dye sensitized solar cell efficiency | DYES AND PIGMENTS | English | Article | Phthalocyanines; Porphyrins; Metal complexes dyes; Dye-sensitized solar cells (DSSC); Solar energy | CO-SENSITIZATION; HIGHLY EFFICIENT; ZINC PHTHALOCYANINES; ORGANIC-DYE; ANATASE; ENERGY; COSENSITIZATION; FABRICATION; TITANIUM; NANOPARTICLES | Effective non-symmetrical push-pull oxotitanium phthalocyanine dyes substituted with tert-butylsulfanyl groups as electron donors and carboxyethynyl units as anchor groups have been designed, prepared, and used with YD2 porphyrin as sensitizer cocktail dyes in dye-sensitized solar cells (DSSCs). The innovative phthalocyanine structures and YD2 and dye cocktails are designed to harvest a broad range from sunlight. The novel phthalocyanine dyes have been characterized via 1H NMR, FTIR, MALDI-TOF, UV-Vis absorption, CV, SWV and TD-DFT techniques. Moreover, the TiO2 NPs has been synthesis by hydrothermal procedure and characterized via XRD, FE-SEM, Raman and DRS techniques. The photoanode has been prepared with synthesized anatase TiO2 NPs using a spin coating procedure. TiO2 photoanode, dye/dye cocktails, I -/I3- electrolyte and Pt/FTO counter electrode have been utilized in the preparation of solar cells. The characterization of the prepared solar cells has been achieved via J-V, Nyquist, Bode, IPCE, normalized JSC and stability measurements. The highest conversion efficiency of the solar cell prepared with YD2:TiOSPPc (3:1) has been performed 10.96%, which this value was found to be higher than the solar cell efficiency of N719 standard dye (8.90%). With these innovative dye cocktails, it is expected that the dye system with not only high efficiency but also a relatively stable and higher VOC value will be an alternative to ruthenium-based dyes. | [Arslan, Nuray; Yildirir, Seyma Nur; Sevim, Altug Mert; Gul, Ahmet] Istanbul Tech Univ, Dept Chem, TR-34469 Istanbul, Turkiye; [Cakar, Soner] Zonguldak Bulent Ecevit Univ, Dept Chem, TR-67100 Zonguldak, Turkiye; [Cakar, Soner; Ozacar, Mahmut] Sakarya Univ, BIOENAMS R&D Grp, Biomat Energy Photocatalysis Enzyme Technol Nano &, TR-54050 Sakarya, Turkiye; [Ozacar, Mahmut] Sakarya Univ, Dept Chem, TR-54050 Sakarya, Turkiye | Istanbul Technical University; Zonguldak Bulent Ecevit University; Sakarya University; Sakarya University | G?l, A i˜A—’˜ŽÒjAIstanbul Tech Univ, Dept Chem, TR-34469 Istanbul, Turkiye. | ahmetg@itu.edu.tr | ; GUL, AHMET/LXW-1956-2024; ?akar, Soner/AAH-1477-2020; Sevim, Altu?/Y-4197-2018; ?zacar, Mahmut/AAF-9122-2020 | Ozacar, Mahmut/0000-0002-1783-7275; Arslan, Nuray/0000-0002-5972-2476; Suerkan, Seyma Nur/0009-0006-1926-9351; | Scientific and Technological Research Council of Turkiye (TUBITAK) [119Z082]; Turkish Academy of Sciences (TUBA) | Scientific and Technological Research Council of Turkiye (TUBITAK)(Turkiye Bilimsel ve Teknolojik Arastirma Kurumu (TUBITAK)); Turkish Academy of Sciences (TUBA)(Turkish Academy of Sciences) | This investigation has been supported by the Scientific and Technological Research Council of Turkiye (TUBITAK) (Project number: 119Z082). AG and MO received partial support from Turkish Academy of Sciences (TUBA). | 71 | 7 | 7 | 3 | 48 | ELSEVIER SCI LTD | London | 125 London Wall, London, ENGLAND | 0143-7208 | 1873-3743 | DYES PIGMENTS | Dyes Pigment. | DEC | 2023 | 220 | 111623 | 10.1016/j.dyepig.2023.111623 | http://dx.doi.org/10.1016/j.dyepig.2023.111623 | SEP 2023 | 12 | Chemistry, Applied; Engineering, Chemical; Materials Science, Textiles | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry; Engineering; Materials Science | T8ZV9 | 2025-09-12 | WOS:001080821500001 | View Full Record in Web of Science | ||||||||||||||||||||||||||
| Yang, LN; Lin, LG; Men, AL; Li, ZJ | Yang, Li-Na; Lin, Li-Guang; Men, A-Lan; Li, Zhen-Jiang | Theoretical insights into co-sensitization mechanism in Zn-porphyrin and Y123 co-sensitized solar cells | JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY | English | Article | Co-sensitization; Zn-porphyrin; Light harvesting; Interfacial electron transfer kinetics | RESONANCE ENERGY-TRANSFER; ORGANIC-DYES; REGENERATION PROCESS; EFFICIENCY; DONOR; COSENSITIZATION; RECOMBINATION; PERFORMANCE; KINETICS | Donor-pi-acceptor YD2-o-C8, WW-6, and SM315 are among best-performing Zn-porphyrins for their outstanding light-harvesting properties. To reach the further enhancement of photovoltaic performance of the corresponding cells, co-sensitization of these typical porphyrins with those dyes possessing intense absorption in the green spectral region (typically, Y123) is necessary. In this work, using density functional theory (DFT) and time dependent DFT (TD-DFT) approaches, co-sensitization mechanism in the above three typical Y123/Zn-porphyrin systems has been systematically investigated. Moreover, due to the excellent performance of dithienosilole (DTS) group in organic dyes, it is designed here to replace the benzothiadiazole (BTD) unit in SM315 to give a new Zn-porphyrin dye designated SM315-1. This specific modification of SM315 can effectively retard the interfacial charge recombination compared to that in YD2-o-C8 and WW-6 cells by reducing the contact between oxidized porphyrins and injected electrons in TiO2 substrate, and simultaneously improves the overall light harvesting ability of the co-sensitized film with the theoretical maximum limit of photocurrent to be 38.31 mA/cm(2). The both favorable aspects suggest an attractive application of the fully novel Y123/SM315-1 co-sensitized film in dye-sensitized solar cell (DSSC). | [Yang, Li-Na; Lin, Li-Guang; Men, A-Lan] Qingdao Univ Sci & Technol, Coll Chem & Mol Engn, State Key Lab Base Ecochem Engn, Qingdao 266042, Shandong, Peoples R China; [Li, Zhen-Jiang] Qingdao Univ Sci & Technol, Coll Sinogerman Sci & Technol, Coll Electromech Engn, Key Lab Polymer Mat Adv Mfg Technol Shandong Prov, Qingdao 266061, Shandong, Peoples R China | Qingdao University of Science & Technology; Qingdao University of Science & Technology | Li, ZJ i˜A—’˜ŽÒjAQingdao Univ Sci & Technol, Coll Sinogerman Sci & Technol, Coll Electromech Engn, Key Lab Polymer Mat Adv Mfg Technol Shandong Prov, Qingdao 266061, Shandong, Peoples R China. | zhenjiangli@qust.edu.cn | Li, Zhenjiamg/S-4826-2017; Meng, Alan/AAI-2946-2020 | National Natural Science Foundation of China [51672144, 51572137, 51502149, 51702181]; Natural Science Foundation of Shandong Province [ZR2016EMB25, ZR2017PEM006, ZR2017BB013]; Higher Educational Science and Technology Program of Shandong Province [J16LA10, J17KA014]; Application Foundation Research Program of Qingdao [15-9-1-28-jch]; Taishan Scholars Program of Shandong Province [ts201511034]; Overseas Taishan Scholars Program | National Natural Science Foundation of China(National Natural Science Foundation of China (NSFC)); Natural Science Foundation of Shandong Province(Natural Science Foundation of Shandong Province); Higher Educational Science and Technology Program of Shandong Province; Application Foundation Research Program of Qingdao; Taishan Scholars Program of Shandong Province; Overseas Taishan Scholars Program | We own our sincere thanks to Prof. Ze-Sheng Li (Beijing Institute of Technology) for his progressive advice about this subject. The work reported here was supported by the National Natural Science Foundation of China under Grant No. 51672144, 51572137, 51502149, 51702181, the Natural Science Foundation of Shandong Province under Grant No. ZR2016EMB25, ZR2017PEM006, ZR2017BB013, the Higher Educational Science and Technology Program of Shandong Province under Grant No. J16LA10, J17KA014, the Application Foundation Research Program of Qingdao under Grant No. 15-9-1-28-jch, the Taishan Scholars Program of Shandong Province under No. ts201511034 and the Overseas Taishan Scholars Program. We express our grateful thanks to them for their financial support. We acknowledge National Supercomputing Center in Shenzhen for providing the computational resources and materials studio (version 6.1, module Dmol3). We also acknowledge National Supercomputing Center of TianHe-II in LvLiang, China, for calculations of part of this work. | 49 | 18 | 19 | 7 | 107 | ELSEVIER SCIENCE SA | LAUSANNE | PO BOX 564, 1001 LAUSANNE, SWITZERLAND | 1010-6030 | 1873-2666 | J PHOTOCH PHOTOBIO A | J. Photochem. Photobiol. A-Chem. | JAN 15 | 2019 | 369 | 25 | 33 | 10.1016/j.jphotochem.2018.10.014 | http://dx.doi.org/10.1016/j.jphotochem.2018.10.014 | 9 | Chemistry, Physical | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry | HG4RP | 2025-09-12 | WOS:000454963000004 | View Full Record in Web of Science | |||||||||||||||||||||||||||
| Hajizadeh, F; Reisi-Vanani, A; Azar, YT | Hajizadeh, Fatemeh; Reisi-Vanani, Adel; Azar, Yavar T. | Theoretical design of Zn-dithiaporphyrins as sensitizer for dye-sensitized solar cells | CURRENT APPLIED PHYSICS | English | Article | Zinc dithiaporphyrin; Porphyrin; Dye sensitized solar cells (DSSC); DFT; TD-DFT; Molecular design | DENSITY-FUNCTIONAL THEORY; PORPHYRIN SENSITIZERS; ZINC PORPHYRINS; PHOTOVOLTAIC PROPERTIES; EFFICIENCY ENHANCEMENT; ELECTRONIC-STRUCTURES; CONDUCTION-BAND; CHARGE-TRANSFER; ENERGY-LEVELS; CHROMOPHORES | We have designed zinc dithiaporphyrin structures based on donor-pi-acceptor (D-pi-A) strategy and studied their optoelectronic properties as sensitizer for dye sensitized solar cells (DSSC) applications. The geometries, HOMO-LUMO energy gap, electronic absorption spectra and light harvesting efficiency (LHE) of these sensitizers were investigated by density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. Our results showed that LUMO energies of all dyes are above the conduction band (CB) of TiO2 and the HOMO energies of them are below the redox couple of I-/I-3(-), thus new sensitizers have convenient HOMO and LUMO energy levels for electron transfer from the excited dye to TiO2 semiconductor and dye regeneration. They also have broadened and red-shifted absorption bands. Open-circuit voltage (V-oc) and the short-circuit current density (J(sc)) parameters including LHE, electron injection driving force (Delta G(inject)) and the free energy change for dye regeneration (Delta G(rege)(n)) were calculated and discussed. The excited and ground state dipole moments were also calculated and their differences (Delta mu(EX-GS)) were discussed. Finally, results showed that thiophene and benzothiadiazole rings in comparison with phenyl ring as at-bridge and cyanoacrylic acid relative to the carboxylic acid as acceptor and anchoring groups showed better efficiency. A general comparison of our new sensitizers with reference dyes (YD2-o-C8 and SM315) showed that new sensitizers can be used for DSSC applications. | [Hajizadeh, Fatemeh; Reisi-Vanani, Adel] Univ Kashan, Fac Chem, Dept Phys Chem, Kashan, Iran; [Azar, Yavar T.] Nucl Sci & Technol Res Inst, Phys & Accelerators Sch, Tehran, Iran | University Kashan | Reisi-Vanani, A i˜A—’˜ŽÒjAUniv Kashan, Fac Chem, Dept Phys Chem, Kashan, Iran. | areisi@kashanu.ac.ir | ; Azar, Yavar/AAE-6981-2020; Reisi-Vanani, Adel/H-3141-2015 | Taghipour Azar, Yavar/0000-0002-9838-5425; Reisi-Vanani, Adel/0000-0003-2716-8623 | University of Kashan [573596/4] | University of Kashan(University Kashan) | The authors are grateful to the University of Kashan for supporting this work by Grant No. 573596/4. | 82 | 18 | 18 | 3 | 103 | ELSEVIER SCIENCE BV | AMSTERDAM | PO BOX 211, 1000 AE AMSTERDAM, NETHERLANDS | 1567-1739 | 1878-1675 | CURR APPL PHYS | Curr. Appl. Phys. | OCT | 2018 | 18 | 10 | 1122 | 1133 | 10.1016/j.cap.2018.06.011 | http://dx.doi.org/10.1016/j.cap.2018.06.011 | 12 | Materials Science, Multidisciplinary; Physics, Applied | Science Citation Index Expanded (SCI-EXPANDED) | Materials Science; Physics | GN5AT | 2025-09-12 | WOS:000439055200005 | View Full Record in Web of Science | |||||||||||||||||||||||||
| S?erkan, SN; Arslan, N; G?k?e?ren, AT; ?akar, S; Sevim, AM; G?l, A; ?zacar, M | Suerkan, Seyma Nur; Arslan, Nuray; Gokceoren, Argun Talat; Cakar, Soner; Sevim, Altug Mert; Gul, Ahmet; Ozacar, Mahmut | A3B type Zn(II) phthalocyanines and porphyrin cocktail dye sensitizers for highly efficient DSSCs | JOURNAL OF PHOTOCHEMISTRY AND PHOTOBIOLOGY A-CHEMISTRY | English | Article | Phthalocyanine; Porphyrin; Sensitizer; Dye-sensitized solar cell; Cocktail dye; DFT | SOLAR-CELL; OSCILLATOR-STRENGTH; ZINC PHTHALOCYANINE; CO-SENSITIZATION; MOLECULAR DESIGN; TIO2; COMPLEXES; LIGHT; SUBSTITUENTS; ENHANCEMENT | Dye-sensitized solar cell (DSSC) technology has recently seen some drastic advancement by new concepts and tailor-made new materials. Phthalocyanines and porphyrins were the most investigated solar sensitive dyes. It has been determined that push-pull phthalocyanines containing carboxylic acid groups are among the promising photosensitizers for dye sensitized solar cells (DSSCs) with their absorption spectra in the NIR region. In particular, carboxylic acid-substituted non-symmetrical metallophthalocyanines appear to be extremely important for electron injection into the TiO2 conduction band in applications where DSSCs have significant potential to achieve greater efficiency. Zinc phthalocyanines containing these groups appear to be candidate molecules for DSSC. However, there is no apparent research in the literature regarding the syntheses and recommendations on analogues of zinc phthalocyanines containing three t-butylsulfanyl or ferrocenylphenol groups and mono aliphatic or aromatic alkynyl anchoring carboxylic acid groups as sensitizing agents for dye-sensitized solar cells. In this actual paper, zinc phthalocyanines and YD2 porphyrin macrocycles were combined as dye cocktails, to extend and complement the absorbance window of sensitizer dyes in the visible region. Thus, four novel A3B type non-symmetrical zinc phthalocyanines have been synthesized and characterized via spectroscopic (1H NMR, FTIR, UV-Vis, MALDI-TOF, etc.), electrochemical (CV, SWV), and molecular (TD-DFT) analysis methods. The reported novel solar sensitive dye-coated cells have been characterized in detail by electrochemical methods i.e., J-V, IPCE and measurements of stability. YD2:ZnPc3 (3:1) cocktail dye yielded the highest conversion efficiency, accomplishing 10.87 % exceeding the respective individual dye counterparts, but also the well-known Ru-based commercial N719 sensitizer's (8.90 %) solar cell efficiencies. | [Suerkan, Seyma Nur; Arslan, Nuray; Gokceoren, Argun Talat; Sevim, Altug Mert; Gul, Ahmet] Istanbul Tech Univ, Dept Chem, TR-34469 Maslak Istanbul, Turkiye; [Cakar, Soner] Zonguldak Bulent Ecevit Univ, Dept Chem, TR-67100 Zonguldak, Turkiye; [Cakar, Soner; Ozacar, Mahmut] Sakarya Univ, Environm Applicat & Sustainabil Res & Dev Grp BIOE, Addit Mfg, Biomat Energy Photocatalysis,Enzyme Technol,Nano &, TR-54187 Sakarya, Turkiye; [Ozacar, Mahmut] Sakarya Univ, Fac Sci, Dept Chem, TR-54050 Sakarya, Turkiye | Istanbul Technical University; Zonguldak Bulent Ecevit University; Sakarya University; Sakarya University | Sevim, AM i˜A—’˜ŽÒjAIstanbul Tech Univ, Dept Chem, TR-34469 Maslak Istanbul, Turkiye. | sevim@itu.edu.tr | ?zacar, Mahmut/AAF-9122-2020; ?akar, Soner/AAH-1477-2020; Gokceoren, Argun/ABH-3036-2020; Sevim, Altu?/Y-4197-2018 | Ozacar, Mahmut/0000-0002-1783-7275; | Scientific and Technological Research Council of Turkey (TUBITAK) [:119Z082]; Turkish Academy of Sciences (TUBA) | Scientific and Technological Research Council of Turkey (TUBITAK)(Turkiye Bilimsel ve Teknolojik Arastirma Kurumu (TUBITAK)); Turkish Academy of Sciences (TUBA)(Turkish Academy of Sciences) | Acknowledgement This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) (Project number:119Z082) . A.G. and M. O. thank Turkish Academy of Sciences (TUBA) for partial support. | 88 | 0 | 0 | 12 | 13 | ELSEVIER SCIENCE SA | LAUSANNE | PO BOX 564, 1001 LAUSANNE, SWITZERLAND | 1010-6030 | 1873-2666 | J PHOTOCH PHOTOBIO A | J. Photochem. Photobiol. A-Chem. | JUL 1 | 2025 | 464 | 116333 | 10.1016/j.jphotochem.2025.116333 | http://dx.doi.org/10.1016/j.jphotochem.2025.116333 | FEB 2025 | 14 | Chemistry, Physical | Science Citation Index Expanded (SCI-EXPANDED) | Chemistry | X5G1P | 2025-09-12 | WOS:001425622900001 | View Full Record in Web of Science |
日本語版
TD-DFTで読み解くZnポルフィリン/YD2系色素
:主要12論文ダイジェスト
概要(3–6文)
本セットは、YD2-o-C8を含むZnポルフィリン色素のDFT/TD-DFT(時間依存密度汎関数理論)計算に基づく設計指針を整理しました。マクロ環・アンカー基・πブリッジ・置換基設計が、**電荷移動(CT)強度、吸収断面積、ΔE(HOMO–LUMO)、光収穫効率(LHE)**などに与える影響を比較し、共増感(co-sensitization)やカクテル色素の有効性も理論面から検討しています。デバイス側のローカル電場や界面整合の影響を扱う研究も含まれ、高効率DSSCに向けた「分子設計 × 界面設計」の要点が俯瞰できます。
ここがポイント(3–6項目)
- マクロ環/アンカー基の入替でCT特性・吸収帯を制御(カルボン酸/ホスホン酸など)。
- **πブリッジ(ヘテロ環・チオ化など)**により可視~近赤での吸収強化とLHE向上を狙う。
- YD2-o-C8, SM315, Y123 等の比較で一線/三重項経路や共増感の相補性を整理。
- ローカル電場・界面整合が電子注入・再結合に与える影響を理論評価。
- カクテル/共増感(ポルフィリン×フタロシアニン等)によりスペクトル補完とJsc向上を期待。
論文別ハイライト(入力順・各2–4行)
- Theoretical study of novel porphyrin-based dye for efficient DSSC(2017)
- 設計/材料:新規ポルフィリン色素 vs 参照(YD2系)をDFT/TD-DFTで比較。
- 機能/現象:CT帯域と吸収断面積の増強。
- 数値:N/A。用途:高LHE志向の分子設計。
- Macrocycle/anchoring group replacements… DFT & TD-DFT(2015)
- 設計/材料:マクロ環・アンカー基の置換効果(YD2-o-C8参照)。
- 機能/現象:吸収シフト、注入能と再生(regeneration)への影響。
- 数値:N/A。用途:アンカー設計の指針。
- Charge Transfer Enhancement in D-π-A Porphyrins(2016)
- 設計/材料:D-π-A型ポルフィリン2種をTD-DFTで比較。
- 機能/現象:CT強度・電荷分離効率の起源を解析。
- 数値:N/A。用途:ブリッジ最適化。
- High-efficient porphyrin-sensitizer under local electric field(2015)
- 設計/材料:界面ローカル電場を考慮した理論検討。
- 機能/現象:電場が注入・再結合に及ぼす影響を示唆。
- 数値:N/A。用途:電極・電解質設計と連携。
- A3B型Zn(II)/TiO(IV)ナフチル色素:Ru代替候補(2023)
- 設計/材料:非対称A3Bポルフィリン/フタロシアニン。
- 機能/現象:吸収補完と注入安定性の追求。
- 数値:記事番号 114642。用途:コスト低減型DSSC。
- YD2-o-C8, SM315, Y123の比較(2016)
- 設計/材料:代表的高効率ポルフィリンの電子構造・励起特性。
- 機能/現象:励起子局在・CT割合の違いを定量比較。
- 数値:N/A。用途:参照系のベンチマーキング。
- Predicting larger absorption cross-section in porphyrins(2015)
- 設計/材料:吸収断面積を増やす置換設計。
- 機能/現象:π共役拡張とドナー強化の効果。
- 数値:N/A。用途:広帯域集光化。
- Zn porphyrin with heterocyclopentadiene π-bridge(2022)
- 設計/材料:ヘテロ五員環ブリッジでの吸収・電荷移動制御。
- 機能/現象:HOMO/LUMO調整とCT増強。
- 数値:N/A。用途:近赤寄りの感光強化。
- S-bridged oxo-Ti phthalocyanine / porphyrin cocktail(2023)
- 設計/材料:チオ架橋フタロシアニン+ポルフィリンの共増感。
- 機能/現象:スペクトル補完と光電流の底上げ。
- 数値:記事番号 111623。用途:高ηカクテルDSSC。
- Co-sensitization mechanism: Zn-porphyrin × Y123(2018)
- 設計/材料:Zn-ポルフィリンとY123の相互作用を理論解析。
- 機能/現象:吸着形態・相互クエンチ・補完吸収のバランス。
- 数値:N/A。用途:共増感の合理設計。
- Zn-dithiaporphyrins design for DSSC(2018)
- 設計/材料:硫黄置換(ジチア)ポルフィリン。
- 機能/現象:吸収スペクトル・LHE・ギャップ調整。
- 数値:N/A。用途:赤側シフト設計。
- A3B Zn(II) phthalocyanines & porphyrin cocktail(2025)
- 設計/材料:A3Bフタロシアニン+ポルフィリンのカクテル。
- 機能/現象:カルボン酸基導入で吸着・注入を両立。
- 数値:記事番号 116333。用途:高効率・低希少材DSSC。
用語ミニ解説(3–6)
- TD-DFT(時間依存密度汎関数理論, Time-Dependent DFT):吸収スペクトルや遷移特性の第一原理計算法。
- D-π-A構造(Donor-π-Acceptor):ドナー/アクセプタをπ共役で結ぶ色素骨格。
- LHE(Light-Harvesting Efficiency):光収穫効率。吸収断面積と関係。
- 共増感(co-sensitization):複数色素でスペクトル補完・再結合抑制を狙う設計。
- アンカー基:TiO₂表面への固定基(–COOH, –PO₃H₂等)。
想定アプリケーション(3–6)
- 高効率・広帯域DSSC(屋内発電・BIPV)
- 低照度での発電最適化(吸収補完設計)
- 希少金属フリー色素設計の探索指針
- 界面電場を意識した電極・電解質共設計
関連キーワード(約10)
YD2-o-C8, Zn-porphyrin, TD-DFT, D-π-A, co-sensitization, cocktail dyes, anchoring group, π-bridge, LHE, charge transfer, HOMO/LUMO
English version
Title
TD-DFT Insights into Zn-Porphyrin / YD2-Type Sensitizers: A 12-Paper Digest
Overview
This set distills DFT/TD-DFT design principles for Zn-porphyrin dyes, including YD2-o-C8 references. We compare how macrocycle/anchoring groups and π-bridges tune charge-transfer strength, absorption cross-section, HOMO–LUMO gap, and light-harvesting efficiency (LHE). Several papers analyze co-sensitization/cocktail strategies (porphyrin × phthalocyanine) and interfacial/local-field effects, bridging molecular design and device-level optimization for high-efficiency DSSCs.
Why it matters / Key points
- Macrocycle/anchor replacement modulates CT and spectral position (e.g., –COOH, –PO₃H₂).
- π-bridge engineering (heterocycles, sulfuration) enhances visible–NIR absorption and LHE.
- Benchmarks across YD2-o-C8, SM315, Y123 clarify complementary roles in co-sensitization.
- Local electric fields and level alignment influence injection/recombination at the interface.
- Cocktail/co-sensitization expands spectral coverage and can boost Jsc.
Highlights by study (input order; 2–4 lines each)
- Theoretical study of novel porphyrin dye for efficient DSSC (2017) — DFT/TD-DFT comparison vs reference dyes (YD2 family); stronger CT bands and larger absorption cross-sections. Metrics: N/A. Use: high-LHE design.
- Effects of macrocycle/anchoring replacements (2015) — Anchor/macrocycle swaps (w/ YD2-o-C8 reference) tune absorption and regeneration/injection. Metrics: N/A. Use: anchor selection.
- CT enhancement in D-π-A porphyrins (2016) — TD-DFT resolves origins of stronger CT and separation. Metrics: N/A. Use: bridge optimization.
- Local-field impact on porphyrin sensitizer (2015) — Interfacial fields affect injection/recombination. Metrics: N/A. Use: electrolyte/electrode co-design.
- Unsymmetrical A3B Zn(II)/TiO(IV) naphthyl dyes (2023) — Porphyrin/phthalocyanine alternatives to Ru dyes; spectral complementarity. Article No.: 114642.
- Comparative study: YD2-o-C8, SM315, Y123 (2016) — Electronic structures/excitations benchmarked by DFT; localization and CT fractions contrasted. Metrics: N/A.
- Predicting larger absorption cross-section (2015) — Substitution patterns to boost absorption; donor strength/conjugation extension matter. Metrics: N/A.
- Zn porphyrin with heterocyclopentadiene π-bridge (2022) — Bridge engineering tunes HOMO/LUMO and CT; toward red-shifted response. Metrics: N/A.
- S-bridged oxo-Ti phthalocyanine/porphyrin cocktail (2023) — Co-sensitization improves coverage and current. Article No.: 111623.
- Co-sensitization mechanism: Zn-porphyrin × Y123 (2018) — Adsorption motifs and mutual quenching vs spectral complementarity analyzed. Metrics: N/A.
- Zn-dithiaporphyrins for DSSCs (2018) — Sulfur-substituted macrocycles: spectra/LHE/gap engineering. Metrics: N/A.
- A3B Zn(II) phthalocyanines & porphyrin cocktail (2025) — Carboxyl anchors aid binding/injection; cocktail strategy emphasized. Article No.: 116333.
Mini-glossary
- TD-DFT (Time-Dependent DFT): First-principles method for excited-state/absorption analysis.
- D-π-A architecture: Donor–π–acceptor conjugated dyes for CT-enhanced injection.
- LHE (Light-Harvesting Efficiency): Proxy linked to absorption cross-section.
- Co-sensitization / cocktail: Multiple dyes to extend spectra and suppress recombination.
- Anchoring group: Surface-binding moiety to TiO₂ (e.g., carboxylate, phosphonate).
Potential applications
- High-efficiency, broadband DSSCs (indoor/BIPV).
- Low-illuminance energy harvesting via spectral complementarity.
- Rare-metal-free sensitizer exploration.
- Interface-aware electrode/electrolyte co-design.
Suggested tags
YD2-o-C8, Zn-porphyrin, TD-DFT, D-π-A, co-sensitization, cocktail dyes, anchoring group, π-bridge, LHE, charge transfer, HOMO/LUMO
参考文献(日本語・英語共通の一覧:入力順)
- Lee, GH, Kim, YS. Theoretical study of novel porphyrin-based dye for efficient dye-sensitized solar cell. Molecular Crystals and Liquid Crystals 2017, 645(1), 168–174. DOI: https://doi.org/10.1080/15421406.2016.1277636
- Shalabi, AS, El Mahdy, AM, Taha, HO, Soliman, KA. The effects of macrocycle and anchoring group replacements on the performance of porphyrin-based sensitizer: DFT and TD-DFT study. Journal of Physics and Chemistry of Solids 2015, 76, 22–33. DOI: https://doi.org/10.1016/j.jpcs.2014.08.002
- Kang, GJ, Song, C, Ren, XF. Charge Transfer Enhancement in the D-π-A Type Porphyrin Dyes: Density Functional Theory (DFT) and Time-Dependent DFT (TD-DFT) Study. Molecules 2016, 21(12), 1618. DOI: https://doi.org/10.3390/molecules21121618
- Xie, M, Wang, J, Ren, J, Hao, L, Bai, FQ, Pan, QJ, et al. Theoretical study on a high-efficient porphyrin-sensitizer in a local electric field: how the local electric field affects the performance of dye-sensitized solar cells? Organic Electronics 2015, 26, 164–175. DOI: https://doi.org/10.1016/j.orgel.2015.07.045
- Demirci, YC, Şakar, S, Sevim, AM, Gül, A, Özacar, M. DSSCs based on unsymmetrical A3B type Zn(II) and TiO(IV) naphthyl dyes: A potential alternative for ruthenium-based sensitizers. Journal of Photochemistry and Photobiology A: Chemistry 2023, 437, 114642. DOI: https://doi.org/10.1016/j.jphotochem.2023.114642
- Li, XY, Zhang, CR, Yuan, LH, Zhang, ML, Chen, YH, Liu, ZJ. A comparative study of porphyrin dye sensitizers YD2-o-C8, SM315 and Y123 for dye-sensitized solar cells: the electronic structures and excitation-related properties. European Physical Journal D 2016, 70(10), 211. DOI: https://doi.org/10.1140/epjd/e2016-70071-3
- Yasin, A, Jose, R, Yusoff, MM. Predicting larger absorption cross-section in porphyrin dyes using DFT calculations. Journal of Porphyrins and Phthalocyanines 2015, 19(12), 1270–1278. DOI: https://doi.org/10.1142/S1088424615501138
- Gao, QQ, Yuan, QT, Song, XF, Yu, YM, Guo, J. Theoretical Study on Regulation of Photoelectric Properties of Zinc Porphyrin Dyes with Heterocyclopentadiene as π-Bridge. Chinese Journal of Inorganic Chemistry 2022, 38(2), 295–303. DOI: https://doi.org/10.11862/CJIC.2022.018
- Arslan, N, Yildirir, SN, Sevim, AM, Şakar, S, Özacar, M, Gül, A, et al. Sulfur-bridged oxotitanium phthalocyanine- and porphyrin-based cocktail dyes as sensitizers for improved dye-sensitized solar cell efficiency. Dyes and Pigments 2023, 220, 111623. DOI: https://doi.org/10.1016/j.dyepig.2023.111623
- Yang, LN, Lin, LG, Men, AL, Li, ZJ. Theoretical insights into co-sensitization mechanism in Zn-porphyrin and Y123 co-sensitized solar cells. Journal of Photochemistry and Photobiology A: Chemistry 2018, 369, 25–33. DOI: https://doi.org/10.1016/j.jphotochem.2018.10.014
- Hajizadeh, F, Reisi-Vanani, A, Azar, YT. Theoretical design of Zn-dithiaporphyrins as sensitizer for dye-sensitized solar cells. Current Applied Physics 2018, 18(10), 1122–1133. DOI: https://doi.org/10.1016/j.cap.2018.06.011
- Şerkan, SN, Arslan, N, Gökçüeren, AT, Şakar, S, Sevim, AM, Gül, A, Özacar, M. A3B type Zn(II) phthalocyanines and porphyrin cocktail dye sensitizers for highly efficient DSSCs. Journal of Photochemistry and Photobiology A: Chemistry 2025, 464, 116333. DOI: https://doi.org/10.1016/j.jphotochem.2025.116333
チ